div class=”ChapterContextInformation”>
13. Role of Non-transecting Anastomotic Urethroplasty for Bulbar Urethral Strictures
Keywords
UrethraBulbusStrictureUrethroplastyAnatomyErectile dysfunction13.1 Introduction
Every surgeon should attempt to reduce iatrogenic damage to surrounding structures and their vasculature during surgical intervention. Excision and primary anastomosis (EPA) is a well-established and frequently used type of urethroplasty in the repertoire of the reconstructive urologist to treat a urethral stricture. Transecting EPA (tEPA), the classic technique, encompasses full transection of the urethra and the surrounding spongious tissue containing the urethral vasculature at the site of the stricture. However, as the spongiofibrosis surrounding the stricture rarely affects the whole thickness of the corpus spongiosum, the question arises whether it is necessary to perform this full thickness transection and its associated iatrogenic damage to the urethra’s blood supply. For this reason, the concept of non-transecting anastomotic urethroplasty (NTAU) was introduced by Jordan et al. in 2007 [1] and further explored in the following decade. To understand the concept of NTAU, it is indispensable to have a profound knowledge of the vascular anatomy of the male urethra. This will be highlighted in this chapter. This chapter will further discuss the indications and limitations of NTAU as well as the theoretic advantages of the technique. A detailed description of the technique and its variants will be provided. The surgical and functional results of currently available series will be summarized.
13.2 Anatomy of the Male Urethra with Special Attention to the Vasculature
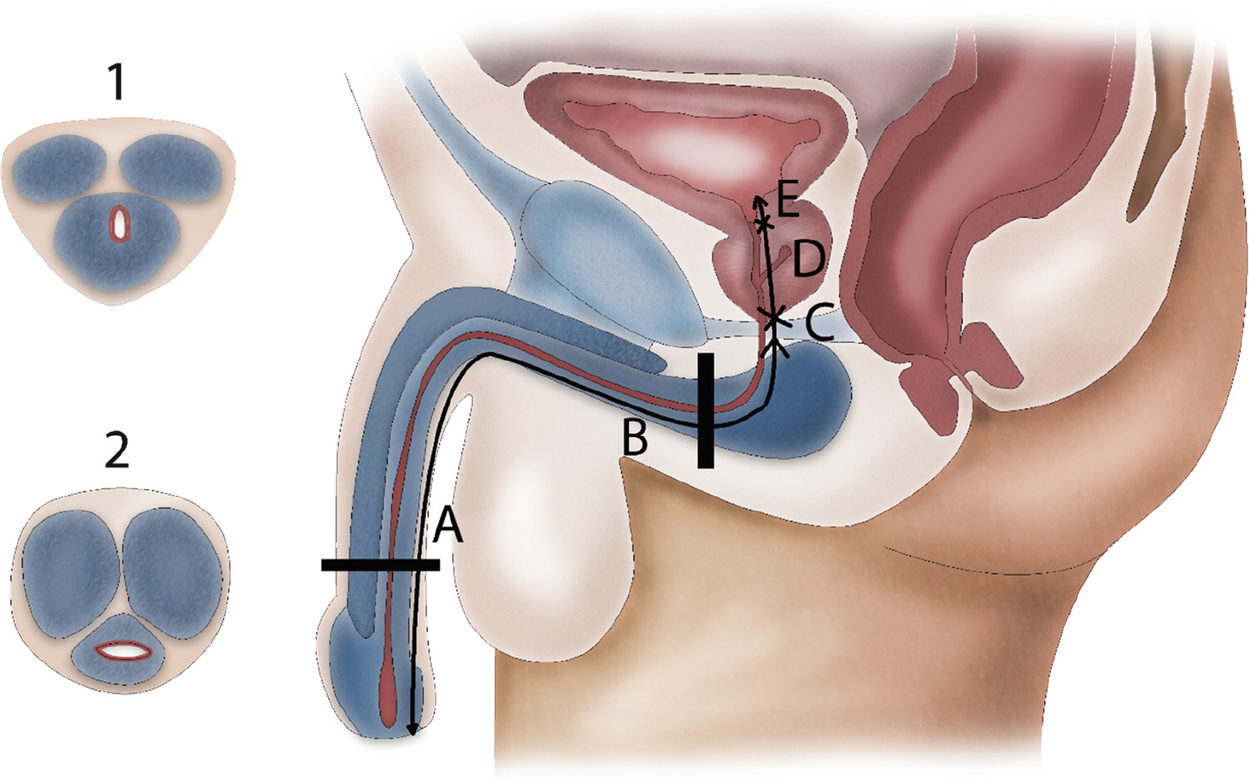
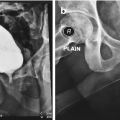
Macroscopic anatomy of the male urethra. The anterior urethra contains the penile (a) and the bulbar (b) urethra. The posterior urethra contains the membranous urethra (c), the prostatic urethra (d) and the bladder neck (e). Transverse section through the bulbus where the urethra lies eccentric in the spongious tissue (1) and the penile part where it is located central in thin spongious tissue (2)
The anterior urethra is surrounded by the corpus spongiosum. At the level of the penile urethra, the corpus spongiosum is relatively thin and the urethra runs centrally through it. This is in contrast to the bulbar area, where the urethra lies eccentric in the corpus spongiosum with abundant ventral spongious tissue. In the bulbar area, the corpus spongiosum is surrounded by the M. bulbospongiosus which is fixed to the urogenital diaphragm by the centrum tendineum or perineal body.
- 1.
the bulbourethral (or bulbar and urethral) artery proximally penetrates the bulbus and is responsible for the antegrade urethrospongiosal blood flow.
- 2.
the dorsal penile artery penetrates the glans penis and as the glans is the distal end of the corpus spongiosum, the dorsal penile artery is responsible for the retrograde urethrospongiosal blood flow.
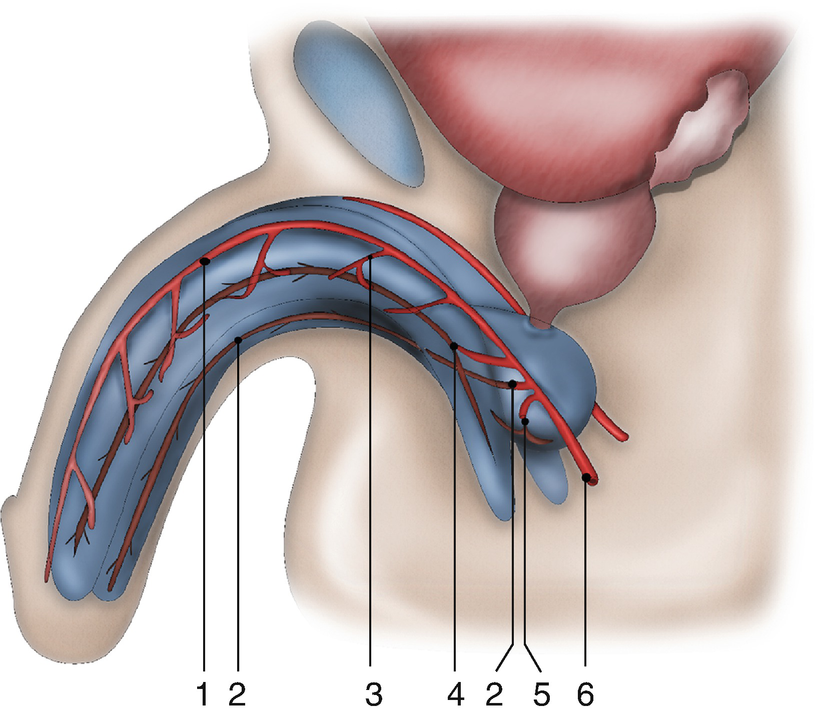
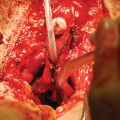
Vascular anatomy of the penis and urethra: (1) dorsal penile artery (2) urethral artery (3) circumflex cavernosal artery (4) central cavernosal artery (5) bulbar artery (6) common penile artery
In addition, there is a third vascularisation due to connecting vessels from the corpora cavernosa. Distal branches of the central cavernosal arteries also penetrate into the glans and add to the retrograde blood flow mentioned in 2. Furthermore, perforating arteries are present between the corpora cavernosa and corpus spongiosum. These perforating vessels are bilaterally present approximately every centimetre at the bulbar urethra. It is unclear whether these connections with the corpora cavernosa are important to the vascular supply during erection.
13.3 Indications and Limitations of NTAU
The different types of NTAU require mobilization of the urethra and will provoke some urethral shortening. Mobilization of the penile urethra followed by an anastomotic repair will lead to penile shortening and chordee. Therefore, NTAU is not indicated for penile strictures, not even short ones. The only exception is a patient who is already impotent and for whom penile length is not important. At the bulbar urethra mobilization between the penoscrotal angle and urogenital diaphragm will not affect the penile length. The bulbar urethra can be shortened somewhat thanks to two factors: the intrinsic elasticity of the urethra and straightening of the curve of the bulbar urethra [2]. The intrinsic elasticity of the bulbar urethra is about 25% and assuming an average bulbar urethral length of 10 cm [3], a gap of up to 2.5 cm can be bridged at the bulbar urethra.
Stricturoplasty based on the Heineke-Mikulicz principle (longitudinal incision, transverse closure) was first described by Lumen et al. in 2010 using a ventral approach [4]. The dorsal approach was first reported by Andrich et al. in 2012 [5]. This technique is only possible for very short strictures (≤1 cm), not too narrow and without substantial underlying spongiofibrosis [4, 6].
Indications for non-transecting excision and primary anastomosis (ntEPA) are the same as for tEPA: short (1–3 cm) bulbar strictures and membranous strictures. The decision to do an EPA (either transecting or non-transecting) is affected by the length of the bulbar urethra and the location of the stricture within the bulbar urethra. The general rule is: “the more proximal (to the prostate), the longer the stricture that can be treated by EPA”. Therefore, it is sometimes possible to treat a 3 cm deep bulbar stricture with EPA whereas it is not possible to treat a 1.5 cm bulbar stricture close to the penoscrotal angle. As a consequence, the classic stricture length limit of 2–2.5 cm is not strict and depends on the patient’s anatomy and stricture location. Because the stricture is removed and the urethra is replaced by its own healthy tissue, the results of EPA are among the best in urethral surgery [7]. The limit of EPA is whenever a tension-free anastomosis can no longer be achieved. If the stricture has been opened and it is apparent that a tension-free anastomosis will not be possible, conversion towards substitution urethroplasty is needed. For ntEPA, where the urethra is opened at the dorsal side, a dorsal onlay procedure with a graft is usually performed. If the stricture has already been resected and after spatulation a tension-free anastomosis cannot be performed, a modified graft augmented end-to-end anastomosis can be performed (Augmented Non-Transecting Anastomosis <ANTA>). ANTA is also an option in case a dorsal onlay graft urethroplasty is performed but a short nearly obliterative segment is present.
Despite the optimal surgical results, some concerns arose with tEPA because of the reported effect of tEPA on the patient’s sexual function [8, 9]. As an alternative, some experts propose graft urethroplasty even for short bulbar strictures [10].
13.4 Possible Benefits of NTAU
In tEPA, the urethral artery is transected and this sacrifices the urethra’s antegrade blood supply. Furthermore, deep ventrolateral mobilisation during tEPA might also damage the bulbar arteries (or the bulbourethral artery). With ntEPA, the bulbar artery or bulbourethral artery is preserved as dissection at deep ventrolateral surface of the bulb is avoided. During ntEPA, it is attempted to preserve the urethral artery within the corpus spongiosum. However, in case of deep spongiofibrosis that also surrounds the urethral artery, the urethral artery needs to be sacrificed as well. Nevertheless, the ventral spongious tissue can be spared with continuous blood flow through the remaining spongious tissue. The circumferential mobilisation needed for both tEPA and ntEPA (but also for dorsal substitution urethroplasty) will damage the perforating arteries on both sides of the corpus spongiosum and this will have an effect on the urethra’s third blood supply. From a theoretical point of view, the more the blood supply towards the glans is disturbed, the greater the risk of vascular changes in the glans. Clinically, this can be apparent as a cold feeling at the glans, alterations in glans sensitivity and/or a reduced tumescence of the glans during erection. These disturbances have been reported in respectively 1.6, 18.3 and 11.6% of cases after tEPA [11]. As the antegrade blood supply towards the glans is better preserved with NTAU, these alterations might be less frequent.
In the elderly and in patients with cardiovascular co-morbidity (esp. diabetes), a better preservation of the spongiosal blood supply might reduce the risk of subsequent ischemic spongiofibrosis and late stricture formation after EPA. In this perspective, preservation of the bulbourethral artery should be beneficial. Furthermore, diabetes and its inherent microangiopathy delays normal wound healing at the anastomosis and spongious tissue, and is a risk factor of failure of urethroplasty [12]. Any factor that avoids further damage to the spongiosal blood supply, as provided by NTAU, might reduce this risk. In patients with hypospadias, the retrograde blood flow is absent and preservation of the antegrade blood flow should avoid ischemic changes in the distal urethra [13].
A better preservation of the spongiosal blood flow with NTAU might be advantageous in case of redo-urethroplasty as well. Approximately 10% of patients treated by EPA will suffer a recurrence (8). One of the options to treat this recurrence is ventral onlay free graft urethroplasty. A free graft urethroplasty is dependent on the vascularization of the graft bed (i.e. spongious tissue) and this might be better after NTAU compared to tEPA.
Preservation of the bulbourethral artery will cause less atrophy of the bulbus and the spongious tissue and there is less risk of erosion, in case an artificial sphincter or male suburethral sling is needed for urinary incontinence at a later stage (e.g. after radical prostatectomy) [1].
Knowing the vascular connections between the corpus spongiosum and the corpora cavernosa, it is possible that impaired spongiosal vascularization can have an effect on the blood supply of the corpora cavernosa. When the penis is in a flaccid state, this can cause ischemic changes in the cavernosal smooth muscle cells. During erection, reduced blood supply and ischemic changes of the cavernosal smooth muscle cells might lead to diminished tumescence. With tEPA, a temporary decline in erectile function has been reported [14, 15]. Although this is most likely the consequence of damage to the cavernosal nerves during deep circumferential dissection, reduced cavernosal blood supply may add to this neurogenic etiology. As deep circumferential dissection is avoided with NTAU, the risk of neurogenic damage should be reduced as well.
It is important to stress that all the above-mentioned benefits are to date purely hypothetical and a direct benefit of NTAU compared to tEPA or other techniques of urethroplasty needs to be elucidated.
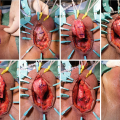
13.5 Surgical Description of Different Techniques of NTAU
- 1.
Stricturoplasty (Heineke-Mikulicz principle)

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree