Femoroacetabular impingement (FAI) is a mechanical hip disorder defined as abnormal abutment between the femoral head or the femoral head–neck junction and the acetabulum. It was initially described as a distinct physiologic entity by Myers et al,28 who noted abnormal contact between the femoral neck and the acetabular rim in a cohort of patients undergoing periacetabular osteotomy (PAO) to correct hip dysplasia.
 One of the earlier descriptions of this condition was by Smith-Petersen35 in 1936, when a case of malum coxae senilis was described. The case in that original article resembled what we now describe as FAI. Another description of this condition came in 1970s when the term pistol grip deformity was introduced. The latter was a description of aberrant morphologic features of the femoral head and neck on anteroposterior (AP) radiographs of patients with early idiopathic hip osteoarthritis (OA).24
One of the earlier descriptions of this condition was by Smith-Petersen35 in 1936, when a case of malum coxae senilis was described. The case in that original article resembled what we now describe as FAI. Another description of this condition came in 1970s when the term pistol grip deformity was introduced. The latter was a description of aberrant morphologic features of the femoral head and neck on anteroposterior (AP) radiographs of patients with early idiopathic hip osteoarthritis (OA).24
 FAI can be anterior or posterior, with anterior abnormalities being more frequent. The estimated prevalence of FAI in the general population is 10% to 15%38 and is increasingly being recognized as a cause of hip pain in young, active individuals. Repetitive anterolateral impingement produces acetabular articular cartilage delamination, labral disease, and eventually secondary OA. Establishment of a correlation between clinical picture, physical findings, suggestive radiographic structural abnormalities, and positive magnetic resonance angiogram (MRA) is crucial for diagnosis and appropriate treatment of this condition.
FAI can be anterior or posterior, with anterior abnormalities being more frequent. The estimated prevalence of FAI in the general population is 10% to 15%38 and is increasingly being recognized as a cause of hip pain in young, active individuals. Repetitive anterolateral impingement produces acetabular articular cartilage delamination, labral disease, and eventually secondary OA. Establishment of a correlation between clinical picture, physical findings, suggestive radiographic structural abnormalities, and positive magnetic resonance angiogram (MRA) is crucial for diagnosis and appropriate treatment of this condition.
ANATOMY
 Establishing an accurate diagnosis and selecting the optimal surgical treatment strategy relies on a thorough understanding of the pathoanatomy of hip impingement disorders.
Establishing an accurate diagnosis and selecting the optimal surgical treatment strategy relies on a thorough understanding of the pathoanatomy of hip impingement disorders.
 Two different structural impingement types have been described:
Two different structural impingement types have been described:
Femoral-based abnormality (ie, cam impingement) is more common in the young, athletic male16 (M:F ratio = 14:1).38 Cam is a term applied to an eccentric prominence in a rotating mechanism which converts rotary motion into linear motion.26
Acetabular-based abnormality (ie, pincer impingement) is seen more commonly in athletic middle-aged women (M:F ratio = 1:3).38 Pincer comes from the French word meaning “to pinch” (FIG 1).1,13,23
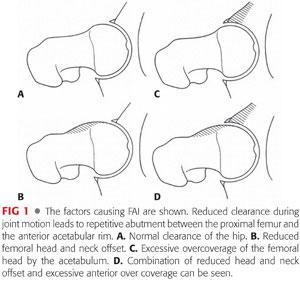
 It is important to note that most FAI cases are likely to be a combination of cam and pincer impingement. In this state, both the proximal femur and the acetabulum present with distorted structural characteristics. Isolated cam or pincer deformities are rare; in 86% of cases, a combined deformity is present.38 In one study of 149 hips with FAI lesions, only 26 hips presented with an isolated cam lesion and 16 hips presented with an isolated pincer lesion.1
It is important to note that most FAI cases are likely to be a combination of cam and pincer impingement. In this state, both the proximal femur and the acetabulum present with distorted structural characteristics. Isolated cam or pincer deformities are rare; in 86% of cases, a combined deformity is present.38 In one study of 149 hips with FAI lesions, only 26 hips presented with an isolated cam lesion and 16 hips presented with an isolated pincer lesion.1
 Cam-type impingement syndrome can be primary (reduced femoral head–neck offset or an aspherical femoral head) with abnormal physeal development or secondary to previous pathologies (eg, slipped capital femoral epiphysis [SCFE], Perthes abnormalities, developmental dysplasia of the hip [DDH], or as a result of a prior trauma to the proximal femur and/or acetabulum).
Cam-type impingement syndrome can be primary (reduced femoral head–neck offset or an aspherical femoral head) with abnormal physeal development or secondary to previous pathologies (eg, slipped capital femoral epiphysis [SCFE], Perthes abnormalities, developmental dysplasia of the hip [DDH], or as a result of a prior trauma to the proximal femur and/or acetabulum).
 Morphologically, cam lesions can result from an osseous bump or from angular deformities of the proximal femur (eg, femoral retrotorsion or coxa vara). The osseous bump location further divides cam lesions into lateral-based prominence (pistol grip) or anterosuperior-based prominence, which is only detected on lateral radiographic views of the hip. Cam impingement owing to femoral angular deformities is uncommon.
Morphologically, cam lesions can result from an osseous bump or from angular deformities of the proximal femur (eg, femoral retrotorsion or coxa vara). The osseous bump location further divides cam lesions into lateral-based prominence (pistol grip) or anterosuperior-based prominence, which is only detected on lateral radiographic views of the hip. Cam impingement owing to femoral angular deformities is uncommon.
 Pincer-type impingement syndrome consists of an overcoverage abnormality, which can be focal or global. Focal overcoverage can be anterior (acetabular retroversion with or without a deficient posterior wall) or posterior (prominent posterior wall). Global overcoverage consists of concentric deepening of the acetabulum (acetabular dome breaching the pelvic brim) which presents as coxa profunda or protrusio acetabuli, with the latter being the most severe form.
Pincer-type impingement syndrome consists of an overcoverage abnormality, which can be focal or global. Focal overcoverage can be anterior (acetabular retroversion with or without a deficient posterior wall) or posterior (prominent posterior wall). Global overcoverage consists of concentric deepening of the acetabulum (acetabular dome breaching the pelvic brim) which presents as coxa profunda or protrusio acetabuli, with the latter being the most severe form.
PATHOGENESIS
 If left untreated, the process of FAI is likely to lead to hip joint degeneration.13,23,39 Mechanical impingement is most noticeable with hip flexion alone or hip flexion combined with internal rotation.
If left untreated, the process of FAI is likely to lead to hip joint degeneration.13,23,39 Mechanical impingement is most noticeable with hip flexion alone or hip flexion combined with internal rotation.
 In pincer impingement, a linear contact occurs between the acetabular overcovering rim with a labrum, which acts like a bumper.1 The head–neck junction, with the maximal impact force tangential to the joint surface,38 leads to a full tear of the labrum, which is the first structure to fail in this situation. Sustained mechanical abutment results in degeneration of the labrum with intrasubstance ganglion formation; ossification of the injured labrum leads to further deepening of the acetabulum and worsening of the overcoverage.24
In pincer impingement, a linear contact occurs between the acetabular overcovering rim with a labrum, which acts like a bumper.1 The head–neck junction, with the maximal impact force tangential to the joint surface,38 leads to a full tear of the labrum, which is the first structure to fail in this situation. Sustained mechanical abutment results in degeneration of the labrum with intrasubstance ganglion formation; ossification of the injured labrum leads to further deepening of the acetabulum and worsening of the overcoverage.24
 In cam impingement, a jamming of the aspherical head portion (shear stress) into the acetabulum occurs, with the maximal impact force perpendicular to the joint surface, leading to an undersurface tear of the labrum fibrocartilaginous separation,38 which more accurately should be called separation of the acetabular cartilage from the labrum.1
In cam impingement, a jamming of the aspherical head portion (shear stress) into the acetabulum occurs, with the maximal impact force perpendicular to the joint surface, leading to an undersurface tear of the labrum fibrocartilaginous separation,38 which more accurately should be called separation of the acetabular cartilage from the labrum.1
 Based on this pathophysiology, the typical location of femoral cartilage damage is circumferential in pincer impingement with contrecoup lesion and is localized between 11 o’clock and 3 o’clock positions in cam impingement.38 A contrecoup lesion is acetabular cartilaginous damage in the opposite part of the femoroacetabular abutment. It is seen in one-third of all cases of pincer impingement and is associated with slight joint subluxation.38 Therefore, the cartilaginous lesions are more benign in pincer impingement than those seen in cam impingement with an average depth of 4 mm in the former and 11 mm in the latter.1,38
Based on this pathophysiology, the typical location of femoral cartilage damage is circumferential in pincer impingement with contrecoup lesion and is localized between 11 o’clock and 3 o’clock positions in cam impingement.38 A contrecoup lesion is acetabular cartilaginous damage in the opposite part of the femoroacetabular abutment. It is seen in one-third of all cases of pincer impingement and is associated with slight joint subluxation.38 Therefore, the cartilaginous lesions are more benign in pincer impingement than those seen in cam impingement with an average depth of 4 mm in the former and 11 mm in the latter.1,38
ETIOLOGY
Genetics
 Numerous studies conducted on family members, especially sets of twins, have demonstrated a genetic component to OA, which is mostly seen in Caucasians. Furthermore, it has been shown that genetic factors are largely responsible for variations in hip and acetabular morphology and cartilage thickness.26
Numerous studies conducted on family members, especially sets of twins, have demonstrated a genetic component to OA, which is mostly seen in Caucasians. Furthermore, it has been shown that genetic factors are largely responsible for variations in hip and acetabular morphology and cartilage thickness.26
 Based on one genetic study, cam deformity tends to be more prevalent in the family members of affected patients than pincer deformity. There is a relative risk of 2.8 and 2, respectively, that a sibling will have the same abnormal anatomy. Likewise, a positive family history increases the risk of bilateralism of the deformity.18
Based on one genetic study, cam deformity tends to be more prevalent in the family members of affected patients than pincer deformity. There is a relative risk of 2.8 and 2, respectively, that a sibling will have the same abnormal anatomy. Likewise, a positive family history increases the risk of bilateralism of the deformity.18
Geographical Variation
 FAI is more prevalent in the Western world where the majority of hip OA, previously labeled primary OA, is currently considered to be the consequence of abnormal hip joint anatomy.
FAI is more prevalent in the Western world where the majority of hip OA, previously labeled primary OA, is currently considered to be the consequence of abnormal hip joint anatomy.
 In contrast, in a retrospective study of 946 primary total hip arthroplasty (THA) conducted in Japan, Takeyama et al37 identified FAI as an underlying pathology in only six hips.
In contrast, in a retrospective study of 946 primary total hip arthroplasty (THA) conducted in Japan, Takeyama et al37 identified FAI as an underlying pathology in only six hips.
Underlying Hip Pathologies
 In some cases, FAI is secondary to childhood hip disorders such as SCFE, DDH, Legg-Calvé-Perthes disease, or femoral neck fracture complicated by malunion.
In some cases, FAI is secondary to childhood hip disorders such as SCFE, DDH, Legg-Calvé-Perthes disease, or femoral neck fracture complicated by malunion.
In one study that followed patients for 15 years, those formerly treated for SCFE subsequently displayed signs of FAI.18
As reported by Eijer et al,9 previous femoral neck fractures may result in secondary FAI, specially if the reduction is not completely anatomic.
Snow et al36 reported four cases of anterior cam impingement which occurred after an asymptomatic period following reossification of the femoral head in Legg-Calvé-Perthes disease.
NATURAL HISTORY
 Because FAI is a newly identified condition, the natural history of its development has not been clearly defined in the literature. However, there is an association between morphologic deformities and secondary OA of the hip.
Because FAI is a newly identified condition, the natural history of its development has not been clearly defined in the literature. However, there is an association between morphologic deformities and secondary OA of the hip.
 Not all patients with FAI will progress to end-stage disease that requires intervention. It is estimated that one-third of patients with mild OA in the presence of FAI will take more than 10 years to develop end-stage OA, if at all.26
Not all patients with FAI will progress to end-stage disease that requires intervention. It is estimated that one-third of patients with mild OA in the presence of FAI will take more than 10 years to develop end-stage OA, if at all.26
 Symptomatic impingement disorders most likely progress to secondary OA, making surgical treatment a rational option.
Symptomatic impingement disorders most likely progress to secondary OA, making surgical treatment a rational option.
 Close observation and follow-up or prompt and timely conservative surgical treatment should be tailored to each case, taking into consideration clinical presentation, radiographic findings, family history, and especially, the rate of disease progression. Clinicians should be aware that delay of surgical correction may lead to chondral damage and disease progression to a stage where joint preservation procedures may be of little benefit.26
Close observation and follow-up or prompt and timely conservative surgical treatment should be tailored to each case, taking into consideration clinical presentation, radiographic findings, family history, and especially, the rate of disease progression. Clinicians should be aware that delay of surgical correction may lead to chondral damage and disease progression to a stage where joint preservation procedures may be of little benefit.26
PATIENT HISTORY AND PHYSICAL FINDINGS
History
 FAI usually affects active young adults and begins with slow onset of activity-related anterior inguinal (groin) pain that is often preceded by a minor traumatic event.24
FAI usually affects active young adults and begins with slow onset of activity-related anterior inguinal (groin) pain that is often preceded by a minor traumatic event.24
 Associated lateral (trochanteric) and posterior hip (gluteal) pain is common but the pain is most commonly felt in the groin (83% of cases).26 The pain is often manifested after sitting for a prolonged period.24
Associated lateral (trochanteric) and posterior hip (gluteal) pain is common but the pain is most commonly felt in the groin (83% of cases).26 The pain is often manifested after sitting for a prolonged period.24
 In the early stages of the disease, the pain or aching is intermittent and could be aggravated by excessive physical demands on the hip, such as prolonged walking or high-demand athletic activities, including running, cutting, pivoting, and repetitive hip flexion.
In the early stages of the disease, the pain or aching is intermittent and could be aggravated by excessive physical demands on the hip, such as prolonged walking or high-demand athletic activities, including running, cutting, pivoting, and repetitive hip flexion.
 Labral disease or unstable articular cartilage flaps3 may cause mechanical symptoms of locking and catching. Anterior FAI is almost always associated with labral tears,5 which are rarely isolated and most likely indicate underlying bony pathologies. Acetabular labral tears should be considered in active patients with a history of groin pain that is exacerbated by activity without radiologic evidence or other hip pathology.3
Labral disease or unstable articular cartilage flaps3 may cause mechanical symptoms of locking and catching. Anterior FAI is almost always associated with labral tears,5 which are rarely isolated and most likely indicate underlying bony pathologies. Acetabular labral tears should be considered in active patients with a history of groin pain that is exacerbated by activity without radiologic evidence or other hip pathology.3
 Stiffness is common, with reductions in the range of hip flexion, adduction, and internal rotation in particular,26 whereas overall hip function is almost unaffected.24
Stiffness is common, with reductions in the range of hip flexion, adduction, and internal rotation in particular,26 whereas overall hip function is almost unaffected.24
 After inquiring about the overall performance of the patient (including age, activity level, and comorbidities), a detailed hip-focused history should be conducted to determine any trauma, childhood hip disease, previous surgeries and treatments as well as the impact of hip pain on quality of life.
After inquiring about the overall performance of the patient (including age, activity level, and comorbidities), a detailed hip-focused history should be conducted to determine any trauma, childhood hip disease, previous surgeries and treatments as well as the impact of hip pain on quality of life.
Physical Examination
 After assessment of overall health and body habitus, the hip clinical examination should be done with great care because it provides the most reliable diagnostic information. Physical findings will dictate further tests and necessary management.
After assessment of overall health and body habitus, the hip clinical examination should be done with great care because it provides the most reliable diagnostic information. Physical findings will dictate further tests and necessary management.
 Observation of sitting posture, gait, palpation of the hip, abductor strength testing, careful hip range-of-motion (ROM) assessment, and specific provocative tests should be performed.
Observation of sitting posture, gait, palpation of the hip, abductor strength testing, careful hip range-of-motion (ROM) assessment, and specific provocative tests should be performed.
Anterior FAI commonly provokes discomfort/pain in an upright sitting position, which involves hip flexion greater than 90 degrees.
The gait pattern over short distances and abductor strength are usually normal in the early stages of the disease. A limp, secondary to mild abductor weakness, and positive Trendelenburg test may develop as labral disease and joint degeneration progress.
 Hip ROM testing should be performed carefully with stabilization of the pelvis to accurately define motion end points. Passive flexion is often limited to approximately 90 degrees and reduced compared with the contralateral hip. Internal rotation may be severely restricted to just a few degrees. Hip discomfort is often reproduced at the end points of passive motion.
Hip ROM testing should be performed carefully with stabilization of the pelvis to accurately define motion end points. Passive flexion is often limited to approximately 90 degrees and reduced compared with the contralateral hip. Internal rotation may be severely restricted to just a few degrees. Hip discomfort is often reproduced at the end points of passive motion.
 Surgical scars from any previous procedures are inspected to aid in planning of any subsequent surgery. When in doubt, infection should be ruled out.
Surgical scars from any previous procedures are inspected to aid in planning of any subsequent surgery. When in doubt, infection should be ruled out.
 The anterior impingement test and Patrick test are sensitive maneuvers to detect intrinsic hip disease and usually reproduce hip symptoms in patients with anterior FAI.
The anterior impingement test and Patrick test are sensitive maneuvers to detect intrinsic hip disease and usually reproduce hip symptoms in patients with anterior FAI.
The anterior impingement test, also known as the flexion, adduction, and internal rotation test, is performed by flexing the patient’s hip to 90 degrees, adducting and internally rotating it simultaneously.
Patrick test, which is also known as the flexion, abduction, and external rotation test, is performed by crossing the examined limb on the other in a figure-of-four (the ipsilateral heel resting on the contralateral knee) and by applying downward pressure to the knee. If pain is elicited on the ipsilateral side anteriorly, it is suggestive of a hip joint disorder on the same side. If pain is elicited on the contralateral side posteriorly around the sacroiliac joint, it is suggestive of pain caused by dysfunction in that joint.
Moreover, the distance from the ipsilateral knee to the bed should be noted. The test is positive if this distance is greater than the corresponding measurement on the opposite side, if the contralateral hip is considered normal.26
 For posterior FAI, an impingement test (performed in the prone position) is considered positive if forced external rotation in full extension is reproducibly painful. Posterior impingement results from focal acetabular overcoverage (pincer); it is manifested radiographically by a posterior wall sign where the too prominent posterior acetabular wall runs lateral to the femoral head center (normally, the posterior rim runs approximately through the femoral head center).38
For posterior FAI, an impingement test (performed in the prone position) is considered positive if forced external rotation in full extension is reproducibly painful. Posterior impingement results from focal acetabular overcoverage (pincer); it is manifested radiographically by a posterior wall sign where the too prominent posterior acetabular wall runs lateral to the femoral head center (normally, the posterior rim runs approximately through the femoral head center).38
 A posterior impingement test can also be positive in anterior FAI when the disease progresses and posteroinferior traction osteophytes develop, producing clinical symptoms of posterior impingement in extension.
A posterior impingement test can also be positive in anterior FAI when the disease progresses and posteroinferior traction osteophytes develop, producing clinical symptoms of posterior impingement in extension.
 The Drehman sign is positive if hip flexion produces unavoidable passive external rotation of the hip.38
The Drehman sign is positive if hip flexion produces unavoidable passive external rotation of the hip.38
 Logroll test is also a useful provocative test in patients with FAI. With the patient in supine position and with the hip and knee in full extension, the foot on the affected extremity is externally rotated. Positive sign is present if this maneuver results in pain in the anterior hip of the rotated extremity.
Logroll test is also a useful provocative test in patients with FAI. With the patient in supine position and with the hip and knee in full extension, the foot on the affected extremity is externally rotated. Positive sign is present if this maneuver results in pain in the anterior hip of the rotated extremity.
IMAGING AND OTHER DIAGNOSTIC STUDIES
 Investigation of FAI requires a combination of roentgenographic examination and more sophisticated cross-sectional studies, such as magnetic resonance imaging (MRI)/MRA and sometimes a computed tomography (CT) scan.
Investigation of FAI requires a combination of roentgenographic examination and more sophisticated cross-sectional studies, such as magnetic resonance imaging (MRI)/MRA and sometimes a computed tomography (CT) scan.
Roentgenography
 Is the first-line investigation and ideally may include five views: a supine AP view of the pelvis, an axial cross-table lateral view (surgical profile of Arcelin and Danelius-Miller view), a frog-leg lateral view, a false profile (Lequesne view), and a Dunn-Rippstein view in 45 or 90 degrees of flexion.4,27,31
Is the first-line investigation and ideally may include five views: a supine AP view of the pelvis, an axial cross-table lateral view (surgical profile of Arcelin and Danelius-Miller view), a frog-leg lateral view, a false profile (Lequesne view), and a Dunn-Rippstein view in 45 or 90 degrees of flexion.4,27,31
 The AP and axial cross-table views are crucial and should be taken with the legs in 15 degrees of internal rotation to adjust for femoral antetorsion and fully expose the femoral neck length.
The AP and axial cross-table views are crucial and should be taken with the legs in 15 degrees of internal rotation to adjust for femoral antetorsion and fully expose the femoral neck length.
 Obtaining an AP view in the supine position allows for a direct comparison with intraoperative and immediate postoperative roentgenograms.38 Standing AP views are important to accurately evaluate and follow joint space narrowing and to apply coxometric measurements.
Obtaining an AP view in the supine position allows for a direct comparison with intraoperative and immediate postoperative roentgenograms.38 Standing AP views are important to accurately evaluate and follow joint space narrowing and to apply coxometric measurements.
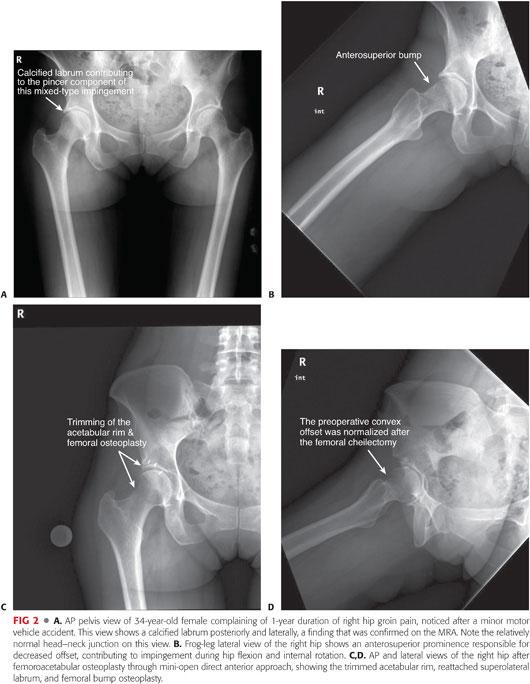
 The AP view of the pelvis mainly detects pincer lesions and laterally based cam lesions (pistol grip). Anterosuperior cam lesion can be missed on AP views and should be investigated on at least one, if not all, of the three lateral views (FIG 2).
The AP view of the pelvis mainly detects pincer lesions and laterally based cam lesions (pistol grip). Anterosuperior cam lesion can be missed on AP views and should be investigated on at least one, if not all, of the three lateral views (FIG 2).
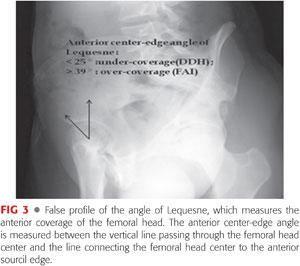
 Lequesne view (false profile) is not used for diagnosing FAI because it does not show the relationship between the anterior and posterior acetabular walls. However, it is more likely used for the diagnosis of early joint degeneration in the posteroinferior part of the acetabulum, which is a relative contraindication for joint-preserving surgery.38 The Lequesne view should be included in a thorough radiologic evaluation to exclude associated mild hip dysplasia because it highlights anterior femoral head coverage (anterior center-edge angle of Lequesne) (FIG 3). Attention should be paid to radiographs where the pelvic tilt, rotation, and inclination are seen. X-ray beam centralization should be taken into consideration for correct interpretation of the radiologic hip parameters. The film-focus distance is different for each view: It should be 120 cm in AP and cross-table views but 102 cm in Dunn, frog-leg, and Lequesne views.38
Lequesne view (false profile) is not used for diagnosing FAI because it does not show the relationship between the anterior and posterior acetabular walls. However, it is more likely used for the diagnosis of early joint degeneration in the posteroinferior part of the acetabulum, which is a relative contraindication for joint-preserving surgery.38 The Lequesne view should be included in a thorough radiologic evaluation to exclude associated mild hip dysplasia because it highlights anterior femoral head coverage (anterior center-edge angle of Lequesne) (FIG 3). Attention should be paid to radiographs where the pelvic tilt, rotation, and inclination are seen. X-ray beam centralization should be taken into consideration for correct interpretation of the radiologic hip parameters. The film-focus distance is different for each view: It should be 120 cm in AP and cross-table views but 102 cm in Dunn, frog-leg, and Lequesne views.38
 The structural parameters of the hip are assessed on all radiographic views (FIGS 4 and 5).
The structural parameters of the hip are assessed on all radiographic views (FIGS 4 and 5).
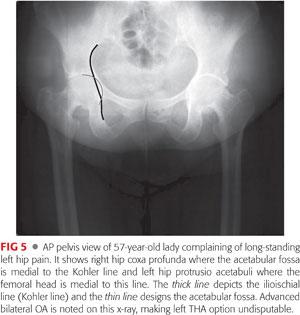
 AP view of the pelvis can identify the following:
AP view of the pelvis can identify the following:
Lateral cam lesion, which is a pistol grip deformity that has a prominent, convex head–neck junction with reduced head–neck offset26 and an aspherical femoral head (alpha angle measurement)
 Acetabular depth, where the acetabular fossa location is noted relative to the Kohler line to look for general acetabular overcoverage. Normally, the fossa is lateral to the Kohler line; coxa profunda is noted when the fossa is medial to this line and protrusio acetabuli when the medial aspect of the femoral head is medial to this line. Acetabular version: look for the following:
Acetabular depth, where the acetabular fossa location is noted relative to the Kohler line to look for general acetabular overcoverage. Normally, the fossa is lateral to the Kohler line; coxa profunda is noted when the fossa is medial to this line and protrusio acetabuli when the medial aspect of the femoral head is medial to this line. Acetabular version: look for the following:
The crossover sign or figure-of-eight sign (first described by Reynolds)26 in anterior acetabular overcoverage (ie, acetabular retroversion)
The prominent wall sign in posterior acetabular overcoverage. Normally, the posterior wall passes approximately through the femoral head center.
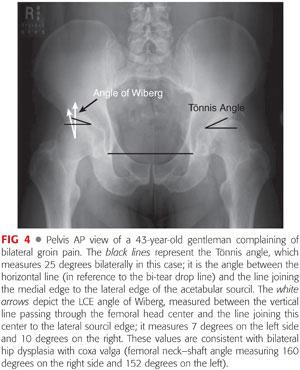
 Often, with so-called acetabular retroversion, the ischial spine is abnormally prominent into the true pelvis medially.38 Acetabular inclination is quantified by the Tönnis angle (acetabular index and acetabular roof angle), which is normally 10 degrees or slightly less.
Often, with so-called acetabular retroversion, the ischial spine is abnormally prominent into the true pelvis medially.38 Acetabular inclination is quantified by the Tönnis angle (acetabular index and acetabular roof angle), which is normally 10 degrees or slightly less.
 Other coxometric measurements noted on the AP view that quantify acetabular depth include the following:
Other coxometric measurements noted on the AP view that quantify acetabular depth include the following:
The lateral center-edge (LCE) of Wiberg: normally varies between 25 and 39 degrees38
The femoral head extrusion index, where the uncovered horizontal portion of the femoral head is quantified, should not exceed 20% to 25%.
 The lateral views are examined to assess the following (FIG 6):
The lateral views are examined to assess the following (FIG 6):
Femoral head sphericity: either by gross visual inspection or using the Mose template. Asphericity can be quantified by measuring the alpha angle of Nötzli (abnormal if >50 degrees in women and >68 degrees in men38). However, this angle measurement is subject to poor intraobserver reliability, with 30% of reliability based on an MRI study.23
• The triangle index has better reproducibility than the alpha angle because it is constructed using clear geometric landmarks and is more independent from femoral rotation.38
Femoral head–neck offset is normally 11.6 ± 0.7 mm.8 Generally, a value inferior to 10 mm is a strong indicator of cam impingement. The offset ratio (femoral offset divided by femoral head diameter) can also be deduced where a value inferior to 0.17 is considered abnormal.21 Indentation sign on the femoral head in pincer impingement.38

 These projections best visualize the anterior and anterolateral femoral head–neck deformity that characterizes cam impingement disease.
These projections best visualize the anterior and anterolateral femoral head–neck deformity that characterizes cam impingement disease.
 Joint space narrowing, periarticular cysts, and labral ossification are also noted on AP and lateral views.
Joint space narrowing, periarticular cysts, and labral ossification are also noted on AP and lateral views.
 The alpha angle and head–neck offset can be measured on the AP view only in the case of pistol grip deformity. Both AP and lateral views should be scrutinized for abnormal morphologic features consistent with FAI.
The alpha angle and head–neck offset can be measured on the AP view only in the case of pistol grip deformity. Both AP and lateral views should be scrutinized for abnormal morphologic features consistent with FAI.
 Secondary changes in impingement can include labral ossification, acetabular stress fracture, and herniation pits (defined as radiolucencies surrounded by a sclerotic margin and located in the anterosuperior quadrant of the femoral neck).
Secondary changes in impingement can include labral ossification, acetabular stress fracture, and herniation pits (defined as radiolucencies surrounded by a sclerotic margin and located in the anterosuperior quadrant of the femoral neck).
Magnetic Resonance Angiogram
 MRA of the hip has been increasingly used in recent years for all patients with suspected impingement and associated intra-articular diseases.
MRA of the hip has been increasingly used in recent years for all patients with suspected impingement and associated intra-articular diseases.
The contour of the femoral head–neck junction,19 labral disease,7 and associated articular cartilage disease (especially in the posteroinferior joint) are better visualized on the MRA. The alpha angle and head–neck offset can be measured more accurately this way.
MRI also excludes other uncommon disorders such as stress fracture, osteonecrosis of the femoral head, neoplasm, infection, and synovial diseases.
 The sensitivity of an MRA is estimated to be 90% with a specificity of 91%.11
The sensitivity of an MRA is estimated to be 90% with a specificity of 91%.11
 Kassarjian et al22 reported that in 88% of cases of symptomatic cam-type impingement, MRA detects a triad of abnormal head–neck morphology, anterosuperior cartilage abnormality, and anterosuperior labral abnormality.
Kassarjian et al22 reported that in 88% of cases of symptomatic cam-type impingement, MRA detects a triad of abnormal head–neck morphology, anterosuperior cartilage abnormality, and anterosuperior labral abnormality.
 Using gadolinium-enhanced MRA, Nötzli et al29 produced the first quantitative study of femoral head asphericity, describing the alpha angle and reporting that values of greater than 55 degrees on average are indicative of FAI.
Using gadolinium-enhanced MRA, Nötzli et al29 produced the first quantitative study of femoral head asphericity, describing the alpha angle and reporting that values of greater than 55 degrees on average are indicative of FAI.
 MRA is the most reliable technique to detect intra-articular lesions and should be performed in conjunction with x-rays whenever FAI is suspected.
MRA is the most reliable technique to detect intra-articular lesions and should be performed in conjunction with x-rays whenever FAI is suspected.
Computed Tomography Scan
 It is the best investigation tool for bone characterization and can be useful in defining the extent of osseous impingement lesions. It shows in detail the contour of the femoral head–neck junction and the exact morphology of the femoral bump and complements other radiologic modalities.
It is the best investigation tool for bone characterization and can be useful in defining the extent of osseous impingement lesions. It shows in detail the contour of the femoral head–neck junction and the exact morphology of the femoral bump and complements other radiologic modalities.
 Alpha angles can be quantified using three-dimensional (3-D) reconstruction of CT scan. The beta angle (the angle at which the posterior aspect of the femoral head becomes aspherical) can also be measured.26
Alpha angles can be quantified using three-dimensional (3-D) reconstruction of CT scan. The beta angle (the angle at which the posterior aspect of the femoral head becomes aspherical) can also be measured.26
 All coxometric measurements (such as acetabular version) can be assessed thoroughly on CT images, confirming the roentgenographic findings.
All coxometric measurements (such as acetabular version) can be assessed thoroughly on CT images, confirming the roentgenographic findings.
DIFFERENTIAL DIAGNOSIS
 Differential diagnosis of FAI is mainly that of groin pain and other pathologies, including multiple hip disorders and pathologies of the adjacent bony and soft tissue structures.
Differential diagnosis of FAI is mainly that of groin pain and other pathologies, including multiple hip disorders and pathologies of the adjacent bony and soft tissue structures.
 Mild hip dysplasia (joint instability) is the first and most important diagnosis to rule out. Usually, most dysplastic hips have excessive acetabular anteversion; however, as stated by Li and Ganz,25 one in six dysplastic hips have some degree of retroversion. This finding should be noted preoperatively to avoid impingement worsening following routine anterior repositioning at the time of PAO.
Mild hip dysplasia (joint instability) is the first and most important diagnosis to rule out. Usually, most dysplastic hips have excessive acetabular anteversion; however, as stated by Li and Ganz,25 one in six dysplastic hips have some degree of retroversion. This finding should be noted preoperatively to avoid impingement worsening following routine anterior repositioning at the time of PAO.
 Isolated intra-articular hip disease (such as villonodular synovitis, chondrocalcinosis, isolated labral tear, chondral disease, and loose body) could explain hip pain in the absence of an impingement deformity.3
Isolated intra-articular hip disease (such as villonodular synovitis, chondrocalcinosis, isolated labral tear, chondral disease, and loose body) could explain hip pain in the absence of an impingement deformity.3
 Extra-articular hip disorders (such as sacroiliac joint disorders, tendinitis, and bursitis) or referred pain (lumbar spine diseases, inguinal hernia, and symptomatic femoral artery aneurysm) should not be overlooked.
Extra-articular hip disorders (such as sacroiliac joint disorders, tendinitis, and bursitis) or referred pain (lumbar spine diseases, inguinal hernia, and symptomatic femoral artery aneurysm) should not be overlooked.
 In cases where multiple disorders coexist or overlap, a diagnostic hip injection can be performed in order to confirm that the hip joint is the pain generator, with complete or near-complete pain relief indicating an intra-articular hip pathology.
In cases where multiple disorders coexist or overlap, a diagnostic hip injection can be performed in order to confirm that the hip joint is the pain generator, with complete or near-complete pain relief indicating an intra-articular hip pathology.
Conservative Treatment
 Nonoperative measures to treat FAI have not been documented in the literature. However, this option should always be considered first. Conservative treatment may help and should be applied as a first-line treatment in symptomatic hips, especially those with mild and intermittent symptoms, before surgery is considered. Treatment may include activity restrictions, hydrotherapy, anti-inflammatory medicines, and intra-articular cortisone injections. The severity of the clinical picture will dictate the eventual therapeutic modalities.
Nonoperative measures to treat FAI have not been documented in the literature. However, this option should always be considered first. Conservative treatment may help and should be applied as a first-line treatment in symptomatic hips, especially those with mild and intermittent symptoms, before surgery is considered. Treatment may include activity restrictions, hydrotherapy, anti-inflammatory medicines, and intra-articular cortisone injections. The severity of the clinical picture will dictate the eventual therapeutic modalities.
 Physical therapy, emphasizing the improvement of passive hip ROM or stretching, is counterproductive and should be avoided because it will irritate the hip and subsequently worsen the pain by sustaining and evolving articular surface damage.
Physical therapy, emphasizing the improvement of passive hip ROM or stretching, is counterproductive and should be avoided because it will irritate the hip and subsequently worsen the pain by sustaining and evolving articular surface damage.
 Anti-inflammatory medicines may be appropriate to relieve acute-onset pain but may also mask the symptoms of an underlying destructive process.23 These medications should be prescribed with caution and administered for a short period of time, given their side effects and taking into consideration symptoms necessitating an extended course of analgesics.
Anti-inflammatory medicines may be appropriate to relieve acute-onset pain but may also mask the symptoms of an underlying destructive process.23 These medications should be prescribed with caution and administered for a short period of time, given their side effects and taking into consideration symptoms necessitating an extended course of analgesics.
 Activity restriction or cessation may alleviate symptoms in some patients. Athletes involved in repetitive hip flexion activities may experience significant relief of discomfort if they refrain from their sport. Although conservative measures are likely to be temporarily successful in some patients, those with a high activity level and athletic ambitions usually have low compliance.23
Activity restriction or cessation may alleviate symptoms in some patients. Athletes involved in repetitive hip flexion activities may experience significant relief of discomfort if they refrain from their sport. Although conservative measures are likely to be temporarily successful in some patients, those with a high activity level and athletic ambitions usually have low compliance.23
SURGICAL MANAGEMENT
 Recognition of the detrimental effect of FAI has led to the development of novel joint-preserving techniques24 aimed at restoring normal structural configurations of the proximal femur and/or acetabulum, interrupting the advancement of osseous morphology, and preventing the development of end-stage OA.
Recognition of the detrimental effect of FAI has led to the development of novel joint-preserving techniques24 aimed at restoring normal structural configurations of the proximal femur and/or acetabulum, interrupting the advancement of osseous morphology, and preventing the development of end-stage OA.
 Corrective surgery can be performed via several modalities, including a mini-open anterior approach, surgical dislocation of the hip,12 combined hip arthroscopy and limited open decompression,5 and arthroscopic decompression alone.15
Corrective surgery can be performed via several modalities, including a mini-open anterior approach, surgical dislocation of the hip,12 combined hip arthroscopy and limited open decompression,5 and arthroscopic decompression alone.15
 Selection of the adequate treatment option depends on multiple factors but is mainly dictated by the type and severity of the underlying abnormal anatomy.
Selection of the adequate treatment option depends on multiple factors but is mainly dictated by the type and severity of the underlying abnormal anatomy.
 Open surgical dislocation of the hip with trochanteric flip osteotomy to perform osteochondroplasty, initially described by Ganz et al12 in 2001, was the mainstay of early surgical management of FAI before the encouraging results of less invasive techniques were published.26
Open surgical dislocation of the hip with trochanteric flip osteotomy to perform osteochondroplasty, initially described by Ganz et al12 in 2001, was the mainstay of early surgical management of FAI before the encouraging results of less invasive techniques were published.26
 Independently of the chosen technique, the primary goal of surgery is to address osseous structural impingement lesions and associated soft tissue intra-articular injuries (eg, labrum or articular cartilage). Many studies have demonstrated more favorable clinical, radiologic, and functional outcomes with labral repair compared to labral débridement in selected cases.2,10,23,32
Independently of the chosen technique, the primary goal of surgery is to address osseous structural impingement lesions and associated soft tissue intra-articular injuries (eg, labrum or articular cartilage). Many studies have demonstrated more favorable clinical, radiologic, and functional outcomes with labral repair compared to labral débridement in selected cases.2,10,23,32
 Surgical dislocation of the hip is reserved for less common cases with nonfocal (global) impingement problems or severe deformities such as advanced nonfocal femoral head deformity encountered in Legg-Calvé-Perthes disease or circumferential pincer impingement where the posterior acetabular rim cannot be exposed via the minimally invasive anterior approach or arthroscopy.
Surgical dislocation of the hip is reserved for less common cases with nonfocal (global) impingement problems or severe deformities such as advanced nonfocal femoral head deformity encountered in Legg-Calvé-Perthes disease or circumferential pincer impingement where the posterior acetabular rim cannot be exposed via the minimally invasive anterior approach or arthroscopy.
 For hips with focal cam-type FAI, the mini-open anterior surgical approach may provide excellent exposure, allowing appropriate correction of the osseous pathology on both sides of the joint while being a minimally invasive procedure.
For hips with focal cam-type FAI, the mini-open anterior surgical approach may provide excellent exposure, allowing appropriate correction of the osseous pathology on both sides of the joint while being a minimally invasive procedure.
Preoperative Planning
 The patient’s history and physical examination findings should be reviewed shortly before proceeding with the surgery, with specific attention drawn to preoperative hip ROM, especially flexion and internal rotation, because these clinical parameters should improve after recontouring of the anterolateral femoral head–neck junction.
The patient’s history and physical examination findings should be reviewed shortly before proceeding with the surgery, with specific attention drawn to preoperative hip ROM, especially flexion and internal rotation, because these clinical parameters should improve after recontouring of the anterolateral femoral head–neck junction.
 Preoperative radiographic studies (x-rays, MRA, and CT scan if obtained) are reevaluated. The size and location of the impingement lesion or lesions are determined, as well as the status of the acetabular labrum and articular cartilage, and are correlated with intraoperative findings.
Preoperative radiographic studies (x-rays, MRA, and CT scan if obtained) are reevaluated. The size and location of the impingement lesion or lesions are determined, as well as the status of the acetabular labrum and articular cartilage, and are correlated with intraoperative findings.
 It is of utmost importance to determine the type (cam, pincer, or a combined cam-pincer) and subtype of the impingement lesion (focal or global, femoral bump or angular deformity) because the characteristics of the specific deformity will dictate the surgical decision making:
It is of utmost importance to determine the type (cam, pincer, or a combined cam-pincer) and subtype of the impingement lesion (focal or global, femoral bump or angular deformity) because the characteristics of the specific deformity will dictate the surgical decision making:
On the acetabular side, the focal or global overcoverage can be corrected by resection osteoplasty of the excessive acetabular brim or by a reverse PAO to reorient a retroverted acetabulum.24 The presence or absence of posterior overcoverage (as detected by the posterior wall sign) as well as the status of the acetabular articular cartilage determines which of these options is elected24; a reverse PAO is preferred if the posterior wall is deficient or the acetabular cartilage is injured.
On the femoral side, an osseous bump is nearly always the culprit lesion in reducing the clearance of the femoral neck during flexion; it can be corrected by resection osteoplasty.
 Although infrequent, proximal femur reorientation osteotomies can be done to address the FAI lesion such as femoral neck lengthening with trochanteric advancement or flexion-valgus intertrochanteric osteotomy (in case of decreased anteversion or varus position of the femoral neck).24
Although infrequent, proximal femur reorientation osteotomies can be done to address the FAI lesion such as femoral neck lengthening with trochanteric advancement or flexion-valgus intertrochanteric osteotomy (in case of decreased anteversion or varus position of the femoral neck).24
 In our practice, osteochondroplasty of the femoral head–neck junction and/or acetabular brim via mini-open anterior approach of the hip is used for the treatment of anterior cam-type impingement and associated intra-articular lesions.
In our practice, osteochondroplasty of the femoral head–neck junction and/or acetabular brim via mini-open anterior approach of the hip is used for the treatment of anterior cam-type impingement and associated intra-articular lesions.
Positioning
 Spinal or general anesthesia can be administered. However, regional anesthesia resulting in optimal muscle relaxation is preferred to facilitate joint distraction.
Spinal or general anesthesia can be administered. However, regional anesthesia resulting in optimal muscle relaxation is preferred to facilitate joint distraction.
 The patient is positioned supine on a regular operating table. We prefer manual distraction of the joint while assessing the central compartment of the hip to minimize the risk of osteonecrosis of femoral head, which is correlated with the duration and force of traction applied through a fracture table.
The patient is positioned supine on a regular operating table. We prefer manual distraction of the joint while assessing the central compartment of the hip to minimize the risk of osteonecrosis of femoral head, which is correlated with the duration and force of traction applied through a fracture table.
 The hip region from the umbilicus to the upper thigh is scrubbed and draped. The whole leg is also scrubbed and draped free to allow unrestricted ROM of the hip during the procedure, a crucial surgical time for the assessment of the adequacy of the osteoplasty.
The hip region from the umbilicus to the upper thigh is scrubbed and draped. The whole leg is also scrubbed and draped free to allow unrestricted ROM of the hip during the procedure, a crucial surgical time for the assessment of the adequacy of the osteoplasty.
TECHNIQUES
 Techniques of Mini-Open Osteochondroplasty through the Direct Anterior Approach
Techniques of Mini-Open Osteochondroplasty through the Direct Anterior Approach
Surgical Approach (Hueter Approach or Smith-Petersen Approach)
A 3- to 4-cm incision is made extending from the lateral aspect of the anterior superior iliac spine heading distally toward the lateral side of the patella (TECH FIG 1A).
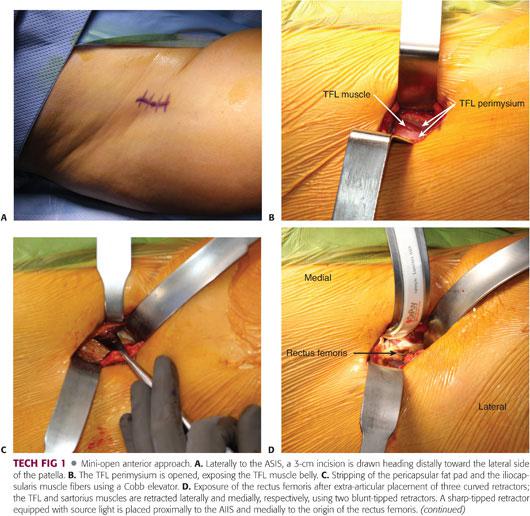
This approach is a muscle-splitting approach, which dissects the interval between the sartorius (innervated by the femoral nerve) and the tensor fascia lata (TFL) (innervated by the superior gluteal nerve). We avoid developing this interval to protect the lateral femoral cutaneous nerve (LFCN) of the thigh.
The dissection is carried through the subcutaneous tissue to the perimysium of the TFL muscle. The subcutaneous dissection is performed slightly lateral to the sartorius–tensor interval. The gap between the sartorius and the TFL can be easily identified and avoided by externally rotating the lower extremity to stretch the sartorius, making it more prominent.
The fascia of the tensor muscle is incised over the muscle belly (TECH FIG 1B), and the TFL muscle is reflected downward and laterally. The medial soft tissue flap, including the tensor perimysium and the sartorius, is reflected upward and medially. The underlying rectus tendon is exposed.
The original deep dissection of this approach involves an internervous passage between the rectus femoris (innervated by the femoral nerve) and the gluteus medius (innervated by the superior gluteal nerve). We do not dissect this interval; instead, we medially retract the rectus femoris without division of any of its insertions to avoid weakness of hip flexion.
After medial retraction of the rectus, the soft tissue and iliocapsularis muscle fibers (iliacus minor, which originates from the anterior inferior iliac spine [AIIS] and inserts into the iliofemoral ligament) are stripped from the anterior hip capsule using a Cobb elevator (TECH FIG 1C).
Blunt-tipped curved retractors are placed, one laterally around the capsule overlying the neck of the femur and one medially retracting the sartorius and rectus muscles to expose the medial capsule of the femoral neck. At this point, the ascending branch of the lateral femoral circumflex artery is ligated or electrocoagulated if it crosses the surgical field.
A third sharp-tipped retractor with a light source is placed at the upper part of the AIIS and under the rectus femoris; the hip should be flexed during placement of this retractor (TECH FIG 1D).
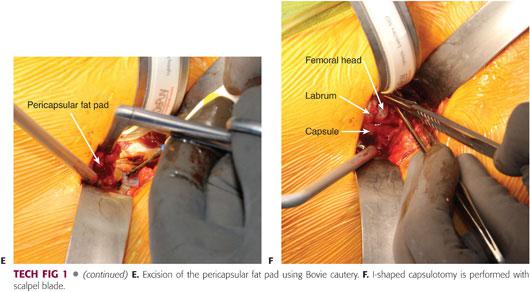
The pericapsular fat pad is grasped with a pituitary instrument and completely excised, giving perfect visualization of the capsule (TECH FIG 1E). The capsule can be stretched and more easily identified by adducting and externally rotating the leg. An “I”-shaped capsulotomy is performed starting with the longitudinal limb (TECH FIG 1F), and the retractors are moved to the intra-articular space to reflect the medial and lateral capsular flaps, providing ample access to the femoral head and neck.
Our mini-invasive anterior approach, which does not detach any muscle insertion, provides excellent access to the joint, which can be inspected with specific attention drawn to the femoral head–neck junction.
Femoral Head–Neck Reshaping Osteoplasty
Based on the preoperative roentgenograms, after adequate exposure of the joint, a thorough assessment of the ROM is done to locate the FAI lesion. This step is crucial in the decision-making process for treatment of FAI.23 The femoral head–neck junction should be inspected while the hip is brought into full and extreme motion. Although impingement in flexion and internal rotation is by far the most common, impingement also may occur in flexion–adduction, flexion–abduction, and rarely in extension and external rotation.23
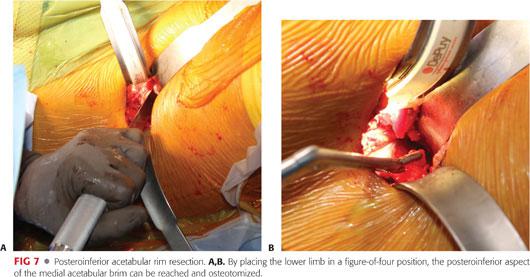
The lower extremity should be brought up into a figure-of-four position to provide adequate visualization of the posteromedial head–neck junction.
The extent of the femoral bump, the presence of labral tear, and cartilaginous lesions are assessed and documented.
There is usually a clear delineation between the normal area of white femoral articular cartilage and the area of the femoral head afflicted with the impingement. The area of FAI (femoral bump) is convex shaped and may be covered by diseased hyaline cartilage, which presents as obvious wear and abnormal creases (indentation sign) created by repetitive contact with the acetabular rim. The femoral bump is often red or blue in appearance, in contrast to the untarnished white hyaline cartilage of the femoral head.23
The labrum should be carefully examined for evidence of tear and/or degeneration. A nerve hook is used to palpate the articular surface of the labrum6 to reveal any tear concealed by the integrity of the capsular aspect of the labrum. Every effort should be made to repair an injured labrum6,23; resection should be reserved only for ossification, attrition, or extensive degeneration and scarring.
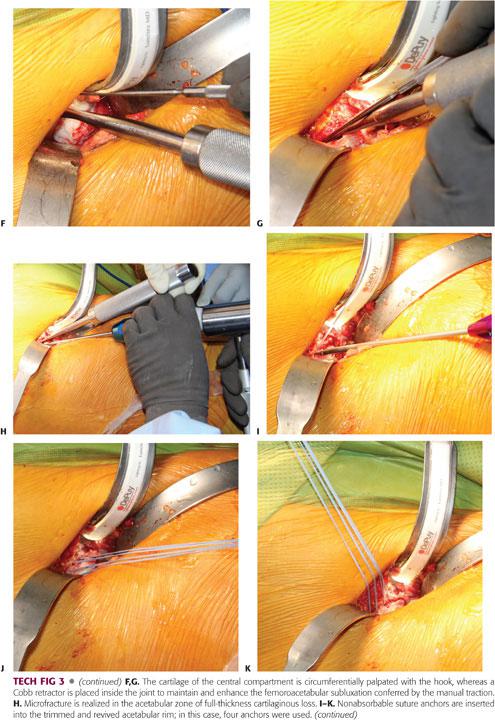
The central compartment of the hip is inspected and palpated by applying a manual traction to the extremity; with the arthrotomy done, minimal traction is sufficient to allow adequate subluxation of the hip and visualization of the weight-bearing dome region of the acetabulum.6 To adequately palpate the cartilage using a hook, the joint subluxation can be maintained and optimized by inserting a smooth Cobb retractor into the joint. Any cartilaginous lesion should be noted and addressed either with conservative débridement, resection of the unstable displaced cartilage flap (which can be responsible for clicking and catching), or microfracture of a full-thickness Outerbridge IV lesion, exposing the underlying subchondral bone.
With the mini-open anterior approach, 60% to 70% of the acetabular cavity can be adequately visualized; the only portion that cannot be exposed is the posteroinferior part of the socket, which can be alternatively palpated by a blunt-tipped nerve hook.6
The osteochondroplasty of the neck can be performed using a combination of osteotomes (TECH FIG 2A) (half- and quarter-inch curved osteotomes) and pneumatic burr (TECH FIG 2B). The femoral bump is located in the anterosuperior region and has a characteristic bluish-reddish discoloration. During this fundamental surgical step, the articular cartilage of the femoral head is protected by releasing the manual traction, allowing the head to re-sit inside the acetabulum.6 If the socket contributes to the impingement lesions, it should be trimmed before proceeding with the femoral osteoplasty.
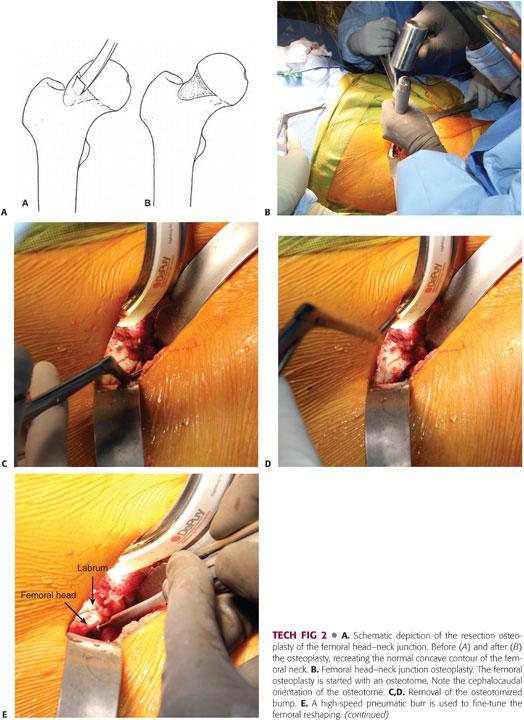
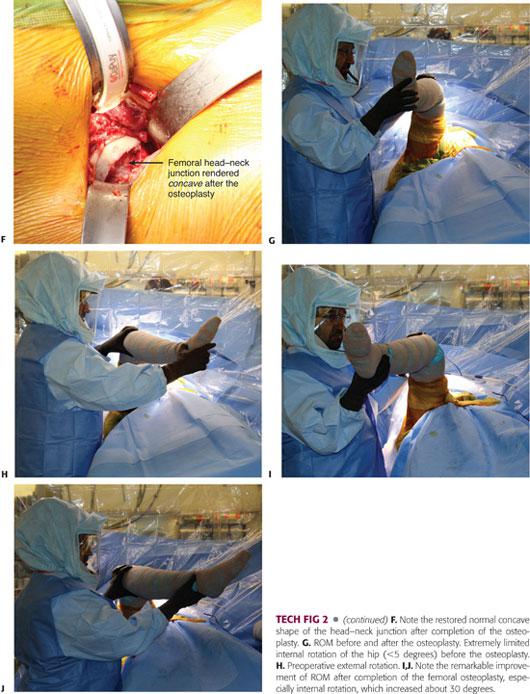
The reshaping of the femoral head–neck junction should begin proximal to the impingement site (TECH FIG 2B) and tapered distally in a near circumferential manner, avoiding the superior portion of the neck where the posterosuperior retinacular vessels enter the bone23; these vessels are the terminal branches of the medial femoral circumflex artery.14
The femoral cheilectomy should proceed in a stepwise manner, resecting at once a small bony sleeve. The adequacy of the resection is dynamically assessed perioperatively by bringing the hip into full ROM. The visualization of the lateral neck is maximized by internal rotation of the hip. The posteroinferior part of the neck is reached by placing the leg in a figure-of-four position.6
If direct visualization and the palpation of the femoral head–neck junction detect residual impingement, the osteoplasty is incrementally refined until it is deemed to be adequate. Ultimately, it should be smoothly beveled inferiorly to prevent notching of the femoral neck.
The extent of bone removal, as well as its depth (which ranges from 5 to 10 mm), is determined by the recreation of the normal smooth concave contour of the head–neck junction and achievement of impingement-free ROM.6 In severe cases, the resection may expand to cover more than 180 degrees of the femoral head–neck circumference.
After completion of the femoral head recontouring, the hip motion should improve at least 10 to 15 degrees in flexion and at least 15 to 20 degrees in internal rotation (TECH FIG 2C), with the concave femoral neck remaining free of abutment with the acetabular brim.23
Fluoroscopic examination can be performed after completion of the cheilectomy, especially at the beginning of the procedure to confirm establishment of head sphericity and adequate osteochondral resection, with special attention paid to match the preoperative views based on the obturator foramen and the ischial tuberosity.
AP and frog-leg lateral views should be obtained to better visualize the anterolateral head–neck junction. Varying degrees of flexion and internal–external rotation permit excellent assessment of the osteochondroplasty. In our practice, where FAI surgical correction is routine, we do not use perioperative fluoroscopic control.
Acetabular Rim Trimming by Resection Osteoplasty
Whether to address the acetabular rim in the treatment of FAI is governed by two essential factors: the existence of anterior focal overcoverage and the condition of the acetabular articular cartilage and the labrum. Taking both factors into consideration will dictate the nature of treatment that should be done on the acetabular side: limited rim osteoplasty versus reverse PAO. If acetabular trimming is deemed necessary to restore normal hip morphology, it should be done before the femoral reshaping.
Acetabular trimming necessitates full exposure of the bony acetabular ridge (TECH FIG 3B); for this purpose, the acetabular labrum must be carefully mobilized. As stated previously, resection of the labrum is done when it is ossified or when it is afflicted with attrition or excessive scarring. If required, the excision of the labrum should be as conservative as possible and limited to the injured area only. Otherwise, a healthy labrum with firm substance or minimally damaged (linear tears) should be preserved and repaired.
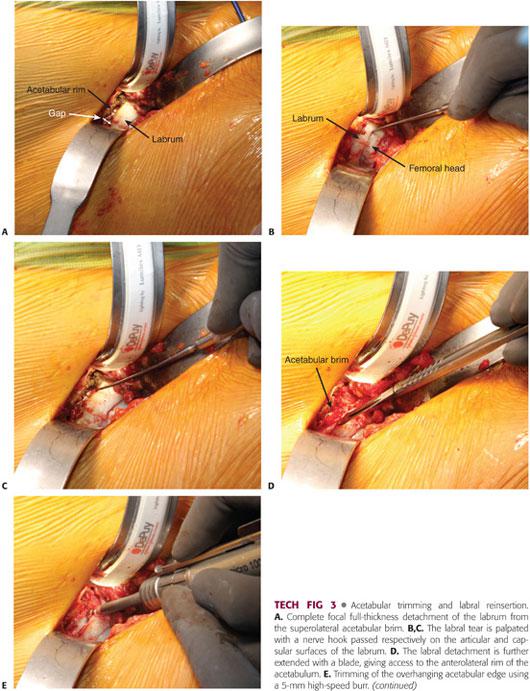
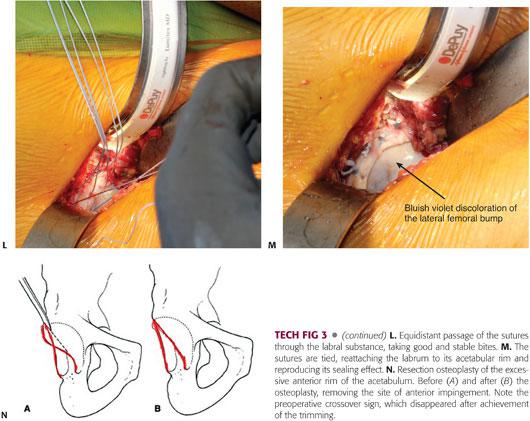
If a focal anterior overcoverage contributes to FAI (acetabular retroversion with a positive crossover sign), a resection osteoplasty of the anterosuperior rim should be performed.
Before the osteoplasty of the rim is accomplished, the labrum, if not torn, is detached cautiously from the anterosuperior acetabular rim using a sharp blade23 while preserving its continuity with the uninvolved normal segment.
Once the bony lip of the acetabulum is adequately exposed, a 10-mm curved osteotome or 5-mm high-speed burr is used to trim the overhanging part of the brim (between 2 and 5 mm).6,23 As for the femoral osteoplasty, the acetabular trimming should be carried out in a stepwise manner until the overcoverage has been entirely resected (TECH FIG 3B).
The amount of rim resection is determined by the magnitude of the anterior overcoverage and extent of the chondral lesion as detected intraoperatively where the area of cartilage damage should be included in the trimming, but excessive (>1 cm) resection should be avoided at all costs to prevent any iatrogenic instability. Roughly 1 mm of resected acetabular edge corresponds to 2 to 3 degrees of correction of the Wiberg angle.
After completion of the acetabular osteoplasty, the labrum is reattached with anchored sutures to the underlying cancellous acetabular rim, which is trimmed down to bleeding bone.6 Three to four anchors are needed to reattach the labrum, and if the acetabular rim is analogous to a clock face, there should be an interval of 1 hour between the two anchors, with 6 hours located at the transverse ligament level.
In cases where the acetabular retroversion is coexistent with a deficient posterior wall (running medial to the femoral head center) or at least lack of posterior overcoverage23 (posterior wall passing through the head center), a reverse PAO should be done after confirming the integrity of the acetabular articular cartilage through MRA. Routine arthrotomy of the hip should always be done at the time of PAO to evaluate the labrum and the articular cartilage for the presence of lesions and to ensure impingement-free hip ROM. However, describing the PAO surgical technique is beyond the scope of this chapter.
 Closure
Closure
After copious irrigation of the hip joint, bone wax is applied to the areas of bone resection on the femoral neck. After a final check of the hip ROM, a meticulous capsulorrhaphy is performed.6
The capsular flaps are reapproximated loosely with an absorbable running suture. Tight closure of the capsule should be avoided because this may compromise the blood supply of the femoral head by placing excessive tension on the retinacular vessels.23 Only the longitudinal limb of the capsulotomy needs to be closed.
The fascia, subcutaneous tissue, and skin are closed in standard fashion (TECH FIG 4).
PEARLS AND PITFALLS | |
Indications |
|
| |
| |
| |
Articular cartilage and labrum |
|
| |
| |
| |
Limited open osteochondroplasty |
|
| |
| |
| |
| |
| |
Postoperative rehabilitation |
|
| |
| |
POSTOPERATIVE CARE
 The patients are usually hospitalized for 24 hours and postoperative radiographs are obtained to verify the adequate recontouring of the femoral head–neck junction and to document the integrity of the femoral neck (see FIG 2). Postoperative films should be compared to the preoperative films, avoiding any pelvic tilt or malrotation.
The patients are usually hospitalized for 24 hours and postoperative radiographs are obtained to verify the adequate recontouring of the femoral head–neck junction and to document the integrity of the femoral neck (see FIG 2). Postoperative films should be compared to the preoperative films, avoiding any pelvic tilt or malrotation.
 Pharmaceutical deep venous thrombosis prophylaxis is treated with aspirin, one tablet of 325 mg administered two times a day for 6 weeks.
Pharmaceutical deep venous thrombosis prophylaxis is treated with aspirin, one tablet of 325 mg administered two times a day for 6 weeks.
 Heterotopic ossification (HO) risk can be minimized with prophylactic administration of anti-inflammatory medications for 6 weeks: celecoxib (400 mg/day) or indomethacin (75 mg/day). The mini-open anterior approach involves little surgical dissection and avoids any muscular detachment, decreasing the risk of HO with minimal blood loss. Copious irrigation of the joint also reduces the risk of HO development.
Heterotopic ossification (HO) risk can be minimized with prophylactic administration of anti-inflammatory medications for 6 weeks: celecoxib (400 mg/day) or indomethacin (75 mg/day). The mini-open anterior approach involves little surgical dissection and avoids any muscular detachment, decreasing the risk of HO with minimal blood loss. Copious irrigation of the joint also reduces the risk of HO development.
 The rehabilitation program consists of touchdown partial weight bearing using crutches for 6 weeks to decrease the risk of femoral neck stress fracture, and then progresses gradually to full weight bearing. During this period, hip flexion is limited to 70 degrees.
The rehabilitation program consists of touchdown partial weight bearing using crutches for 6 weeks to decrease the risk of femoral neck stress fracture, and then progresses gradually to full weight bearing. During this period, hip flexion is limited to 70 degrees.
 To prevent adhesions between the capsule and the osteotomized zone of the femoral neck, continuous passive motion (no more than 70 degrees of hip flexion) can be used for 4 to 6 hours per day for 1 or 2 weeks postoperatively.23
To prevent adhesions between the capsule and the osteotomized zone of the femoral neck, continuous passive motion (no more than 70 degrees of hip flexion) can be used for 4 to 6 hours per day for 1 or 2 weeks postoperatively.23
 Formal physical therapy can be started 6 weeks after surgery. The rehabilitation should consist of a gentle ROM program and progressive strengthening within the patient’s comfort zone. Because the surgical technique does not involve trochanteric osteotomy, abduction strengthening can be initiated early in the postoperative period.
Formal physical therapy can be started 6 weeks after surgery. The rehabilitation should consist of a gentle ROM program and progressive strengthening within the patient’s comfort zone. Because the surgical technique does not involve trochanteric osteotomy, abduction strengthening can be initiated early in the postoperative period.
 The patient may return to normal daily activities (such as walking, ascending or descending stairs, and riding a stationary bicycle) as tolerated 4 to 6 weeks after surgery and may return to full activity once ROM and strength are restored and pain reduced, typically 4 to 6 months postoperatively.
The patient may return to normal daily activities (such as walking, ascending or descending stairs, and riding a stationary bicycle) as tolerated 4 to 6 weeks after surgery and may return to full activity once ROM and strength are restored and pain reduced, typically 4 to 6 months postoperatively.
OUTCOMES
 Because FAI has gained significant interest only during the last decade, only a small number of studies have been published documenting the clinical outcome of conservative surgical treatment.2,10,15,32 Good midterm results have been reported using various surgical approaches for treatment of FAI, including surgical dislocation, arthroscopy, combined arthroscopy, and limited open and direct anterior mini-open approaches.30
Because FAI has gained significant interest only during the last decade, only a small number of studies have been published documenting the clinical outcome of conservative surgical treatment.2,10,15,32 Good midterm results have been reported using various surgical approaches for treatment of FAI, including surgical dislocation, arthroscopy, combined arthroscopy, and limited open and direct anterior mini-open approaches.30
 As surgical dislocation of the hip was the mainstay of treatment for FAI, several studies evaluated this particular surgical option, providing early- to midterm clinical outcomes which are promising.2,10,32,40 Despite its ease of performance and relatively good clinical outcome, dislocation carries a risk of developing complications, mainly HO12 (37%) and greater trochanter malunion (up to 20%).40
As surgical dislocation of the hip was the mainstay of treatment for FAI, several studies evaluated this particular surgical option, providing early- to midterm clinical outcomes which are promising.2,10,32,40 Despite its ease of performance and relatively good clinical outcome, dislocation carries a risk of developing complications, mainly HO12 (37%) and greater trochanter malunion (up to 20%).40
 In addition, although there were no reported cases of avascular necrosis (AVN), in the initial description of the procedure, laser Doppler flowmetry showed transient changes in head perfusion during the procedure, which returned to baseline after reduction of the joint.5 Dislocation also requires the rupture or division of the ligamentum teres with loss of its proprioceptive nerve fibers, the consequences of which are currently unknown.5
In addition, although there were no reported cases of avascular necrosis (AVN), in the initial description of the procedure, laser Doppler flowmetry showed transient changes in head perfusion during the procedure, which returned to baseline after reduction of the joint.5 Dislocation also requires the rupture or division of the ligamentum teres with loss of its proprioceptive nerve fibers, the consequences of which are currently unknown.5
 Arthroscopic osteochondroplasty has certain potential disadvantages, including the risk of inadequate exposure of the anterolateral head–neck junction, entrapment of bony debris within the joint, increasing the risk of HO, and the possibility of inadequate osseous débridement, leading to failed procedure.
Arthroscopic osteochondroplasty has certain potential disadvantages, including the risk of inadequate exposure of the anterolateral head–neck junction, entrapment of bony debris within the joint, increasing the risk of HO, and the possibility of inadequate osseous débridement, leading to failed procedure.
 The mini-open anterior approach combine the advantages of arthroscopic decompression and open surgical dislocation while avoiding the main drawbacks of these two techniques:
The mini-open anterior approach combine the advantages of arthroscopic decompression and open surgical dislocation while avoiding the main drawbacks of these two techniques:
Similar to hip arthroscopy, no traction is required, and the mini-open procedure carries less risk of neural damage and scuffing of the femoral head cartilage.
It is a minimally invasive approach that avoids hip dislocation and the need for trochanteric osteotomy, which is top on the list of complications (trochanteric malunion) associated with this surgical method.
It provides a wide and comprehensive view of all the compartments of the hip, which can be suboptimal in the technically demanding arthroscopic-only procedure, allowing adequate care for chondral and labral lesions.
 Our experience with the mini-open anterior approach has been encouraging when applied to appropriately indicated impingement cases. Analysis of 293 consecutive cases performed in 265 patients between January 2006 and February 2011 revealed good to excellent clinical outcomes; 156 hips (149 patients) achieved a minimum of 2-year follow-up. Out of these, 11 hips underwent THA and 1 hip underwent resurfacing due to degenerative joint disease at an average of 1.4 years postoperatively.30
Our experience with the mini-open anterior approach has been encouraging when applied to appropriately indicated impingement cases. Analysis of 293 consecutive cases performed in 265 patients between January 2006 and February 2011 revealed good to excellent clinical outcomes; 156 hips (149 patients) achieved a minimum of 2-year follow-up. Out of these, 11 hips underwent THA and 1 hip underwent resurfacing due to degenerative joint disease at an average of 1.4 years postoperatively.30
 Despite the auspicious outcome of this technique, it may not be advocated for more advanced disease with posterior impingement lesions or for hips that have circumferential lesions of the femoral head where surgical dislocation, as described by Ganz et al,12 seems to be more accommodating.
Despite the auspicious outcome of this technique, it may not be advocated for more advanced disease with posterior impingement lesions or for hips that have circumferential lesions of the femoral head where surgical dislocation, as described by Ganz et al,12 seems to be more accommodating.
COMPLICATIONS
General Complications
 As with any surgical procedure, this less invasive intervention carries a risk for general complications not directly related to the surgical technique; the most relevant are the following:
As with any surgical procedure, this less invasive intervention carries a risk for general complications not directly related to the surgical technique; the most relevant are the following:
Infection
Deep venous thrombosis
 The relatively short operative time and young age of the patients, along with the fast recovery following the procedure, indicates that the likelihood of developing these complications is exceedingly small.
The relatively short operative time and young age of the patients, along with the fast recovery following the procedure, indicates that the likelihood of developing these complications is exceedingly small.
Specific Complications Related to the Surgical Technique
 The rate and nature of complications associated with the surgical treatment of FAI differ according to the type of surgical procedure performed. The mini-open approach nullifies the risk of trochanteric malunion observed with the surgical dislocation technique and reduces significantly the risk of articular cartilage scuffing, which can occur in hip arthroscopy, especially if the instruments are introduced before obtaining sufficient hip distraction of 8 to 10 mm.
The rate and nature of complications associated with the surgical treatment of FAI differ according to the type of surgical procedure performed. The mini-open approach nullifies the risk of trochanteric malunion observed with the surgical dislocation technique and reduces significantly the risk of articular cartilage scuffing, which can occur in hip arthroscopy, especially if the instruments are introduced before obtaining sufficient hip distraction of 8 to 10 mm.
 Neurovascular injury
Neurovascular injury
The LFCN is at greatest risk of injury through the mini-open approach.6 If the interval between the TFL and the sartorius is dissected, the fascia of the latter should be avoided because the LFCN runs over it. Dissection of this interval is not mandatory to provide wide access to the hip joint.
This approach does not carry the risk of neurologic damage associated with the arthroscopy where
• Excessive hip flexion can injure the sciatic nerve.
• An inadequately padded perineal post can compress the pudendal nerve.
Transient neurapraxia has been reported with the use of excessive traction applied during hip arthroscopy.
The femoral artery and nerve can be injured through both the arthroscopic and open approaches. Following anatomic landmarks carefully during the mini-open anterior approach spares these vital structures.
 HO
HO
Copious irrigation of the joint after the cheilectomy is completed and before proceeding with the capsulorrhaphy decreases the risk of HO. This risk is higher with the purely arthroscopic technique where osteochondroplasty is performed with a high-speed burr, yielding osseous debris; in this case, high-flow hip irrigation along with continuous suction should be used all the time.
In addition to profuse irrigation, prophylactic use of indomethacin minimizes the risk of HO development.
 Femoral neck fracture
Femoral neck fracture
Removing more bone proximally than distally on the head–neck junction can create an apple-bite20 defect instead of the normal concave-shape junction; this defect can get wedged into the acetabular dome during hip flexion, breaking the suction seal of the labrum and increasing the risk of postoperative femoral neck fracture.
In contrast, incomplete reshaping of the head–neck junction is probably more frequent with the purely arthroscopic technique, which has a long learning curve. Several publications have stated that the main reasons for hip arthroscopy revision are inadequate remodeling of FAI deformities.17
The technique described here provides an excellent visualization of the hip and is associated with lesser risk of failure due to inadequate osteoplasty. However, recurrence of the osseous bump can still occur with any technique used to treat FAI; applications of bone wax on the bleeding reshaped bony cuts reduce the risk of bump reappearance.
 AVN of the femoral head
AVN of the femoral head
The risk of AVN following hip arthroscopy was theoretical until the results of two cases were published in the literature.33,34 It is believed that femoral head osteonecrosis may develop secondary to a combination of increased intra-articular pressure and traction.
A constant and reliable landmark to identify the hip blood supply arthroscopically is the lateral synovial fold.17
Because no traction is needed during the mini-open approach, it can be hypothesized that the risk of AVN is less with this procedure, but special care needs to be taken to avoid the zone of the femoral neck where the retinacular vessels run. Because FAI treatment through hip dislocation has not been shown to increase the risk of AVN,12 this risk is small after cheilectomy through an anterior approach, sparing the need for hip dislocation.
In our large series of patients operated on using the mini-open anterior approach, postoperative complications included one neuroma (0.6%) that required excision, one subtrochanteric hip fracture (0.6%) that required open reduction and internal fixation, one repeat labral tear (0.6%) that underwent arthroscopic débridement, and one case of persistent trochanteric bursitis (0.6%) that required iliotibial band lengthening/greater trochanteric bursa excision.30
REFERENCES
1. Beck M, Kalhor M, Leunig M, et al. Hip morphology influences the pattern of damage to the acetabular cartilage: femoroacetabular impingement as a cause of early osteoarthritis of the hip. J Bone Joint Surg Br 2005;87:1012–1018.
2. Beck M, Leunig M, Parvizi J, et al. Anterior femoroacetabular impingement: part II. Midterm results of surgical treatment. Clin Orthop Relat Res 2004;(418):67–73.
3. Burnett RS, Della Rocca GJ, Prather H, et al. Clinical presentation of patients with tears of the acetabular labrum. J Bone Joint Surg Am 2006; 88:1448–1457.
4. Clohisy JC, Keeney JA, Schoenecker PL. Preliminary assessment and treatment guidelines for hip disorders in young adults. Clin Orthop Relat Res 2005;441:168–179.
5. Clohisy JC, McClure JT. Treatment of anterior femoroacetabular impingement with combined hip arthroscopy and limited anterior decompression. Iowa Orthop J 2005;25:164–171.
6. Cohen SB, Huang R, Ciccotti MG, et al. Treatment of femoroacetabular impingement in athletes using a mini-direct anterior approach. Am J Sports Med 2012;40:1620–1627.
7. Czerny C, Hofmann S, Neuhold A, et al. Lesions of the acetabular labrum: accuracy of MR imaging and MR arthrography in detection and staging. Radiology 1996;200:225–230.
8. Eijer H, Leunig M, Mohamed N, et al. Cross table lateral radiographs for screening of anterior femoral head neck offset in patients with femoro acetabular impingement. Hip Int 2001;11:37–41.
9. Eijer H, Myers SR, Ganz R. Anterior femoroacetabular impingement after femoral neck fractures. J Orthop Trauma 2001;15(7):475–481.
10. Espinosa N, Rothenfluh DA, Beck M, et al. Treatment of femoro-acetabular impingement: preliminary results of labral fixation. J Bone Joint Surg Am 2006;88:925–935.
11. Ferguson TA, Matta J. Anterior femoroacetabular impingement: a clinical presentation. Sports Med Arthrosc 2002;10:134–140.
12. Ganz R, Gill TJ, Gautier E, et al. Surgical dislocation of the adult hip: a technique with full access to the femoral head and acetabulum without the risk of avascular necrosis. J Bone Joint Surg Br 2001;83: 1119–1124.
13. Ganz R, Parvizi J, Beck M, et al. Femoroacetabular impingement: a cause for osteoarthritis of the hip. Clin Orthop Relat Res 2003;417:112–120.
14. Gautier E, Ganz K, Krügel N, et al. Anatomy of the medial femoral circumflex artery and its surgical implications. J Bone Joint Surg Br 2000;82:679–683.
15. Guanche CA, Bare AA. Arthroscopic treatment of femoroacetabular impingement. Arthroscopy 2006;22:95–106.
16. Hack K, Di Primio GD, Rakhra K, et al. Prevalence of cam-type femoroacetabular impingement morphology in asymptomatic volunteers. J Bone Joint Surg Am 2010;92(14):2436–2444.
17. Ilizaliturri VM Jr. Complications of arthroscopic femoroacetabular impingement treatment: a review. Clin Orthop Relat Res 2009;467: 760–768.
18. Imam S, Khanduja V. Current concepts in the diagnosis and management of femoroacetabular impingement. Int Orthop 2011;35:1427–1435.
19. Ito K, Minka MA II, Leunig M, et al. Femoroacetabular impingement and the cam-effect. An MRI-based quantitative anatomical study of the femoral head-neck offset. J Bone Joint Surg Br 2001;83:171–176.
20. Jackson T, Stake CE, Trenga AP, et al. Arthroscopic technique for treatment of femoroacetabular impingement. Arthrosc Tech 2013;2(1): e55–e59.
21. Kappe T, Kocak T, Bieger R, et al. Radiographic risk factors for labral lesions in femoroacetabular impingement. Clin Orthop Relat Res 2011;469(11):3241–3247.
22. Kassarjian A, Yoon LS, Belzile E, et al. Triad of MR arthrographic findings in patients with cam-type femoroacetabular impingement. Radiology 2005;236:588–592.
23. Lavigne M, Parvizi J, Beck M, et al. Anterior femoroacetabular impingement: part I. Techniques of joint preserving surgery. Clin Orthop Relat Res 2004;418:61–66.
24. Leunig M, Parvizi J, Ganz R. Nonarthroplasty surgical treatment of hip osteoarthritis. Instr Course Lect 2006;55:159–166.
25. Li PL, Ganz R. Morphologic features of congenital acetabular dysplasia: one in six is retroverted. Clin Orthop Relat Res 2003;(416): 245–253.
26. Macfarlane RJ, Haddad FS. The diagnosis and management of femoro-acetabular impingement. Ann R Coll Surg Engl 2010;92: 363–367.
27. Meyer DC, Beck M, Ellis T, et al. Comparison of six radiographic projections to assess femoral head/neck asphericity. Clin Orthop Relat Res 2006;445:181–185.
28. Myers SR, Eijer H, Ganz R. Anterior femoroacetabular impingement after periacetabular osteotomy. Clin Orthop Relat Res 1999;(363): 93–99.
29. Nötzli HP, Wyss TF, Stoecklin CH, et al. The contour of the femoral head-neck junction as a predictor for the risk of anterior impingement. J Bone Joint Surg Br 2002;84(4):556–560.
30. Parvizi J, Huang R, Diaz-Ledezma C, et al. Mini-open femoroacetabular osteoplasty: how do these patients do? J Arthroplasty 2012;27 (8 suppl):122–125.
31. Peelle MW, Della Rocca GJ, Maloney WJ, et al. Acetabular and femoral radiographic abnormalities associated with labral tears. Clin Orthop Relat Res 2005;441:327–333.
32. Peters CL, Erickson JA. Treatment of femoroacetabular impingement with surgical dislocation and debridement in young adults. J Bone Joint Surg Am 2006;88:1735–1741.
33. Sampson TG. Complications of hip arthroscopy. Clin Sports Med 2001;20:831–835.
34. Scher DL, Belmont PJ Jr, Owens BD. Osteonecrosis of the femoral head after hip arthroscopy. Clin Orthop Relat Res 2010;468: 3121–3125.
35. Smith-Petersen MN. The classic: treatment of malum coxae senilis, old slipped upper femoral epiphysis, intrapelvic protrusion of the acetabulum, and coxa plana by means of acetabuloplasty. J Bone Joint Surg 1936;18:869–880.
36. Snow SW, Keret D, Scarangella S, et al. Anterior impingement of the femoral head: a late phenomenon of Legg-Calvé-Perthes’ disease. J Pediatr Orthop 1993;13(3):286–289.
37. Takeyama A, Naito M, Shiramizu K, et al. Prevalence of femoroacetabular impingement in Asian patients with osteoarthritis of the hip. Int Orthop 2009;33(5):1229–1232.
38. Tannast M, Siebenrock K. Conventional radiographs to assess femoroacetabular impingement. Instr Course Lect 2009;58:203–212.
39. Tanzer M, Noiseux N. Osseous abnormalities and early osteoarthritis: the role of hip impingement. Clin Orthop Relat Res 2004;429: 170–177.
40. Yun HH, Shon WY, Yun JY. Treatment of femoroacetabular impingement with surgical dislocation. Clin Orthop Surg 2009;1:146–154.
< div class='tao-gold-member'>




















