Fig. 2.1
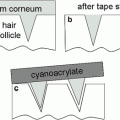
Simplified diagram showing a model for the stratum corneum with various defense lines against penetration of xenobiotics (Modified from Barry 1987)
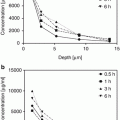
The integrity of human skin is also linked to its water content which varies across skin strata with the concentration of water increasing as we move from the skin surface to deep strata. At the SC, the water concentration is less than 15 %, but increases deeper into the skin where it reaches a five times higher value at the basal skin layers (Warner et al. 1988). This creates a transdermal hydration gradient which is characteristic for normal skin (Cevc and Blume 1992).
2.3 Enhancing Effect of Occlusion on Transdermal Delivery
Skin occlusion can inhibit the continuous water loss from skin surface. This can result in overhydration of the SC with subsequent reduction in the barrier nature of the SC (Bucks et al. 1991). The penetration-enhancing effect of water can be explained on the basis that water forms hydration shells around the head groups of the SC lipids. This leads to the disruption of the closely packed hydrocarbon chains resulting in a loose organization of SC lipids. In addition, excess water can extend the hydrophilic domains of the lamellar structure of the SC lipids providing room for drug permeation (Barry 1987). However, occlusion does not increase percutaneous absorption of all types of drugs. For example, occlusion increased the permeation of citropten (lipophilic compound) 1.6 times, but the transdermal flux of caffeine (amphiphilic compound) was the same under occlusive or open application (Treffel et al. 1992). In a more recent study, the effect of occlusion was shown to depend on the vehicle. In this study, the authors evaluated the penetration of a mixture of paraben ester preservatives from a commercially available test ointment and two commonly employed solvent vehicles (acetone and ethanol), together with the effect of occlusion on the rate of delivery of the preservatives from these systems. Occlusion was achieved by the placement of a piece of high-density polyethylene on the application site immediately after dosing. There was a significant difference in the epidermal flux of parabens provided by the different vehicles applied under occlusion. Whereas increased flux was observed from acetone and ethanol used as vehicles, a decreased flux was seen upon the occlusive application of the ointment formulation (Cross and Roberts 2000). These results can be explained on the basis that occlusion increased the contact time between the skin and the volatile solvents (ethanol and acetone) which possess the penetration-enhancing ability. In contrast, open application of these solvents will lead to their rapid evaporation and hence shorter contact time with the skin and lower penetration-enhancing effect. Open application of these solvents will thus provide a less damaging effect on the skin compared to the occlusive application of the same solvents.
2.4 Delivery Systems Utilizing the Method of Application
2.4.1 TransfersomesTM
While most of the application studies employ mainly occlusive application with no attention paid to the possible effect of the application method, Cevc and Blume (1992) highlighted the importance of the application method on the transdermal delivery of drugs from the highly deformable lipid vesicles, Transfersomes TM . They indicated that these vesicles can penetrate into and through intact skin, carrying therapeutic amounts of drugs if applied under nonocclusive conditions. The ability of such carriers to penetrate intact skin was attributed to their xerophobia (tendency to avoid dry surrounding), which causes the vesicles to resist dehydration at the skin surface by moving into the skin along with the local transdermal hydration gradient (Cevc and Blume 1992; Cevc et al. 1995). Vesicles’ deformation ability was considered as another essential property which made the transdermal hydration gradient to provide sufficient driving force for vesicle penetration through the skin. The vesicles were thus given the name “ultradeformable vesicles” (El Maghraby et al. 2000, 2001a, b; Cevc et al. 2002). Based on this hypothesis, ultradeformable vesicles need to be applied under nonocclusive conditions, as occlusion is believed to abolish the driving force for the skin penetration of vesicles (Cevc and Blume 1992). The same group applied the local anesthetic lidocaine entrapped in Transfersomes TM under occlusion using a watertight wrapping for 25 min over the applied formulation (Planas et al. 1992). The results showed the superiority of Transfersomes TM compared to traditional formulations.
The above hypothesis was not accepted easily by researchers in the field of dermal and transdermal drug delivery. To test this hypothesis, it was important to test occlusive versus nonocclusive application of liposomes. On doing so, it was important to maintain the hydration gradient even in the in vitro experiments. To verify this, El Maghraby and coworkers developed an in vitro experiment which preserves the transepidermal hydration gradient (El Maghraby et al. 1999). This was achieved by the developed open hydration protocol in which the epidermal membrane was hydrated from viable epidermal side with the stratum corneal surface being left open to the atmosphere, to mimic the in vivo conditions. This protocol was employed to study the transdermal delivery of estradiol from ultradeformable liposomes after their occlusive and nonocclusive application (El Maghraby et al. 2001b). Occlusion resulted in a significant reduction of the transdermal flux of the drug which confirms the importance of the application protocol for the transdermal drug delivery from ultradeformable vesicles.
2.4.2 Supersaturable Transdermal Delivery Systems
Supersaturation is the process of creation of a transient, metastable, supersaturated state in which the thermodynamic activity of the drug is increased above unity. This can increase the driving force for transdermal drug delivery. Alternative methods have been adopted to obtain drug supersaturation leading to increased chemical potential and enhanced transdermal drug delivery. The first method was based on mixing of cosolvents for a certain drug, with the rapid addition of a nonsolvent thereby creating a solution in which the drug concentration exceeds its equilibrium solubility (Megrab et al. 1995). In the second method, supersaturation can be achieved by the uptake of water diffusing passively out of the skin into the formulation, in which case water can act as the nonsolvent. This can represent a special case of the first method and can gain the benefit of occlusive application in which the formulation can mix with skin secretion. This process was used to explain the enhanced pharmacodynamic effect of bupranolol after dermal administration using microemulsion (ME) (Kemken et al. 1992). Taking this process into consideration, a self-microemulsifying drug delivery system (SMEDDS) of ethyl oleate, polyoxyethylene 20 sorbitan monooleate (Tween80) and sorbitan monolaurate (Span20), was developed and used to enhance the transdermal delivery of indomethacin compared to the corresponding ME formulation containing increasing concentration of water (El Maghraby 2010). The results indicated the superiority of SMEDDS compared to the tested ME as indicated from the recorded transdermal drug flux values which revealed greater transdermal drug delivery from SMEDDS compared to that obtained from ME. This superiority was attributed to possible supersaturation of the drug after dilution of the SMEDDS with the hydroalcoholic receptor which will diffuse from the receptor upward through the skin, acting as nonsolvent. The author supported this claim by comparing the transdermal delivery of indomethacin from SMEDDS with that obtained from supersaturated microemulsion system which was prepared by diluting saturated drug solution in SMEDDS with water which acts as the nonsolvent, creating supersaturated system before occlusive application to the skin. The results revealed similar flux from the SMEDDS and the prepared supersaturated system.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree