Nonmelanoma skin cancer (NMSC) is the most common form of malignancy in humans. The incidence of NMSC continues to increase despite increased awareness and sun-protective measures. If neglected or mismanaged, NMSC can cause significant morbidity and even death. The most common forms of NMSC on the head and neck include basal cell carcinoma, squamous cell carcinoma, sebaceous carcinoma, eccrine porocarcinoma, Merkel cell carcinoma, atypical fibroxanthoma, and microcystic adnexal carcinoma. Surgery is the mainstay of treatment (standard excision, Mohs micrographic surgery, curettage); however, other modalities exist, including radiation, topical immunomodulators, photodynamic therapy, and new systemic medications.
Key points
- •
Nonmelanoma skin cancer (NMSC) is the most common form of malignancy in humans.
- •
The most common forms of NMSC include basal cell carcinoma, squamous cell carcinoma, sebaceous carcinoma, eccrine porocarcinoma, Merkel cell carcinoma, atypical fibroxanthoma, and microcystic adnexal carcinoma.
- •
Most NMSCs are related to ultraviolet light exposure; other predisposing factors include exposure to radiation, human papillomavirus, immunosuppression, and genetic predisposition.
- •
Surgery (Mohs micrographic surgery, standard excision, or curettage) remains the treatment of choice for most lesions, but other methods exist, including radiation, topical immunomodulators, photodynamic therapy, and new systemic medications.
Introduction
Nonmelanoma skin cancer (NMSC) is the most common form of malignancy in humans and represents nearly 95% of all cutaneous neoplasms. The incidence of NMSC has increased yearly by 3% to 8% since 1960 worldwide. Recent Medicare claims data revealed that more than 3.5 million people in the United States were diagnosed with NMSC in 2006. Because of the current age shift of the US population, the incidence of NMSC may increase by an estimated 50% by 2030. This increase is despite growing public awareness campaigns targeting the harmful effects of ultraviolet (UV) exposure.
Introduction
Nonmelanoma skin cancer (NMSC) is the most common form of malignancy in humans and represents nearly 95% of all cutaneous neoplasms. The incidence of NMSC has increased yearly by 3% to 8% since 1960 worldwide. Recent Medicare claims data revealed that more than 3.5 million people in the United States were diagnosed with NMSC in 2006. Because of the current age shift of the US population, the incidence of NMSC may increase by an estimated 50% by 2030. This increase is despite growing public awareness campaigns targeting the harmful effects of ultraviolet (UV) exposure.
Basal cell carcinoma
Definition, Epidemiology, and Pathogenesis
Basal cell carcinoma (BCC) is a malignant neoplasm of keratinocytes that reside within the basal layer of the epidermis. There is some evidence that the malignant cells may be derived from immature pluripotent cells of the interfollicular epidermis and the outer root sheath of the hair follicle. A definite correlation exists between UV exposure and the genesis of BCC, because there is a higher frequency of disease among patients with history of significant sun exposure. The pattern of exposure (intermittent and intense) and type of UV radiation (UVB) have also been linked to higher BCC rates.
On the molecular level, nearly 90% of all sporadic BCCs have mutations in the hedgehog signaling pathway. The hedgehog pathway is an intracellular signaling cascade key to the regulation of cell growth and differentiation during embryogenesis. The extracellular hedgehog protein binds to a transmembrane receptor, patched homolog 1 (PTCH1), and prevents downstream PTCH1-mediated inhibition of signaling by smoothened homolog (SMO). SMO signaling activates a family of transcription factors encoded by the GLI family zinc finger. Usually inactive in adult tissues, mutations to either PTCH-1 or SMO result in constitutive activation of the hedgehog signaling pathway, thus allowing unrestricted proliferation of epidermal basal cells.
Clinical Findings
BCC is divided clinically into 4 major subtypes: superficial ( Fig. 1 ), nodular ulcerative ( Fig. 2 ), pigmented ( Fig. 3 ), and morpheaform ( Fig. 4 ). The nodular ulcerative subtype is the most common, representing approximately 50% to 85% of all biopsied BCC lesions, followed by superficial, morpheaform, and pigmented forms.
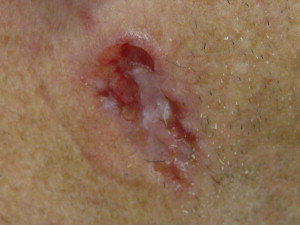
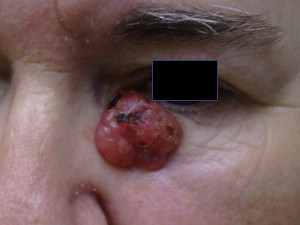
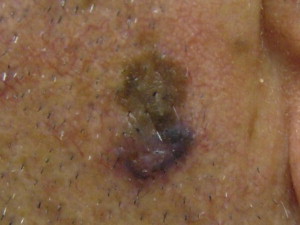
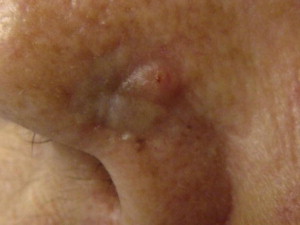
Classically nodular lesions appear as a pearly papule or nodule with visible telangiectasias and an elevated rolled border. As lesions progress, they can centrally ulcerate and, if left untreated, invade critical structures of the head and neck. Superficial BCC lesions present as erythematous smooth to finely scaling patches mainly on the neck and shoulders and are often confused for eczema, superficial squamous cell carcinoma (SCC), or dermatophytosis. Morpheaform lesions often pose the worst prognosis and mainly present on the face as depressed, indurated plaques with ill-defined borders. Pigmented BCCs appear similarly to malignant melanoma and benign pigmented seborrheic keratosis.
Histopathology
Histologic subtype is an important prognostic indicator and should be provided in all biopsy pathology reports. Nearly all clinical subtypes show the diagnostic finding of basaloid, basophilic staining cells budding downward from the epidermis with palisading of peripheral cells ( Fig. 5 ). Tumors become infiltrative when basaloid islands lose their epidermal connections and invade the underlying dermis. Infiltrative growth and perineural invasion portends a more aggressive clinical behavior and increased risk of recurrence.
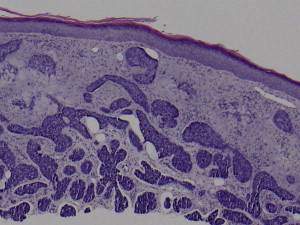
Therapeutic Options
Management of BCC is determined by the lesion size, clinical and histopathologic subtype, depth, site, and patient comorbidities. The treatment of choice for lesions involving the head and neck is Mohs micrographic surgery (MMS), because it provides complete histopathologic examination of all margins of tissue removed. This procedure allows for preservation of healthy tissues in areas at high risk for recurrence (central, periauricular, and periorbital face). In addition, MMS offers the lowest 5-year recurrence rates of all treatment options: primary lesions (1%–2%), secondary lesions (4%–7%). Multiple reviews of the literature support MMS as a more cost-effective treatment of NMSC (including BCC) than standard excision. Other surgical options include standard wide local excision with 4-mm to 5-mm margins for nonaggressive BCC tumors in areas where tissue preservation is unessential. Curettage may be used to treat superficial lesions smaller than 2 cm or for patients who are poor surgical candidates.
Nonsurgical treatment options for BCC lesions include radiation, imiquimod, photodynamic therapy (PDT), ingenol mebutate (PEP005), and vismodegib.
External beam radiation
BCCs are relatively radioresponsive; therefore, radiotherapy can be used as a primary curative modality, surgical adjunctive therapy, or as palliative treatment. Radiation is the primary treatment of choice for patients who are poor surgical candidates.
Imiquimod
Topical imiquimod acts as an agonist of the toll-like receptor 7, which activates the cellular immune response to destroy dysplastic keratinocytes. Imiquimod 5.0% is approved by the US Food and Drug Administration (FDA) to treat nonfacial biopsy-confirmed superficial BCC. Although the use of topical therapy does not confirm margin control, imiquimod monotherapy has shown a 5-year clearance rate as high as 80%.
PDT
PDT involves the activation of a photosensitizing drug (5-aminolevulinic acid, methyl ester form, methyl-5-aminolevulinate) by irradiation with light to create free radicals, resulting in highly targeted tumor cell destruction. PDT is FDA approved only for the treatment of actinic keratoses (AKs); however, many clinical studies support PDT as an effective alternative treatment of superficial BCCs. A large multicenter study by Vinciullo and colleagues showed a 92% response rate for superficial BCCs of the central face. Tumor thickness is a limiting factor for PDT, because the topical medication has limited absorption into the dermis. Therefore, nodular forms of BCC are best served by MMS or simple excision.
Ingenol mebutate (PEP 005)
A natural substance found in the sap of the plant Euphorbia peplus , ingenol mebutate has shown cytotoxic activity against AKs, SCC, and BCC lesions. Recently (2012) approved by the FDA for the treatment of AKs, ingenol mebutate 0.05% gel offers a distinct advantage of shorter treatment duration (2–3 days) compared with imiquimod 5% cream (2–3 months). The proposed mechanism of action involves both neutrophil-mediated cytotoxicity and cellular necrosis of tumor cells. Early phase I/II trials show promising evidence for the use of ingenol mebutate in superficial BCCs. One study reported complete clearance for 57% (16 of 28) of superficial BCC lesions after only 3 consecutive days of treatment. In addition, there were no recurring lesions over the study follow-up period (mean of 15 months).
Vismodegib
Vismodegib was recently approved for the treatment of locally advanced and metastatic BCC. The medication targets gain-of-function mutations within the SMO protein, a member of the hedgehog intracellular signaling pathway. A phase I clinical trial showed an overall 54% response rate, both complete and incomplete, with the oral medication. Side effects are minor, including fatigue, hyponatremia, muscle spasm, and gastrointestinal upset.
SCC
Epidemiology, Definition, and Pathogenesis
SCC is the second most common form of NMSC, with an estimated incidence of nearly 700,000 new cases annually in the United States. SCC accounts for approximately 20% of all NMSC cases, and is the second most common form of cancer in the white population.
A malignant neoplasm of keratinocytes, SCC lesions show full-thickness epidermal dysplasia. SCC may arise de novo or from AKs, premalignant precursor lesions that show partial-thickness epidermal dysplasia. AKs progress to full-thickness malignancy at an estimated rate of 0.025% to 16% for an individual lesion per year. SCC lesions progress from in situ (Bowen disease) to invasive lesions once tumor cells invade the basement membrane of the dermal-epidermal junction.
UVB-induced inactivation of p53, a key tumor-suppressor gene, occurs in nearly 90% of all SCC lesions and 75% to 80% of premalignant AKs. p53 plays an important role in cell cycle arrest at the G1/S checkpoint. Normally the protein plays a fundamental role in the induction of apoptosis after UV light damage, thus preventing the development of malignant keratinocyte populations. Over time, keratinocytes acquire multiple UV-induced genetic mutations, leading to squamous cell dysplasia and subsequent in situ and invasive disease.
Clinical Findings
SCC typically develops on sun-exposed surfaces of the head and neck, including the scalp, ears, midface, lower lip, and neck. AKs, precursor lesions of SCC, present as erythematous scaly or crusted macules and papules. AKs are important markers of UV-induced photodamage, and their presence generally signals an increased risk of NMSC. SCC in situ (Bowen disease) presents as well-defined, erythematous, scaly papules and plaques ( Fig. 6 ). More advanced lesions of SCC present with ill-defined, indurated, scaling papules, plaques, or nodules ( Fig. 7 ). Ulceration and crusting may signal dermal invasion ( Fig. 8 ); therefore, regional lymph nodes should be examined for signs of lymphadenopathy and metastatic disease.
Histopathology
SCC is categorized by the thickness of epidermal invasion and degree of keratinocyte differentiation. In situ disease correlates with full-thickness epidermal involvement ( Fig. 9 ), whereas invasive lesions show dermal invasion. Histologic grading is subdivided into 3 categories depending on the degree of keratinocyte keratinization: well-differentiated, moderately differentiated, and poorly differentiated. The degree of differentiation correlates with tumor aggressiveness, because poorly differentiated SCC lesions have higher recurrence (28.6%) and metastatic (32.8%) rates compared with rates of well-differentiated lesions (13.6%) and (9.2%), respectively. Additional histopathologic features with poor prognostic implications include depth of invasion, desmoplastic reaction around infiltrating keratinocyte islands, and perineural or intravascular invasion.
Therapeutic Options
Multiple treatment modalities, both surgical and nonsurgical, exist for the treatment of SCC. The treatment of choice depends on the characteristics of the patient and their neoplasm. Considerations include the patient’s age and functional status, surgical candidacy, lesion size, location, depth of invasion, level of differentiation, perineural or perivascular invasion, recurrence of previous SCC, and immunosuppression.
Similar to BCC lesions, MMS is the treatment of choice for lesions involving the head and neck, most notably areas with a high risk of recurrence (central face, periorbital, periocular areas). Other indications for MMS include tumors larger than 2 cm, aggressive histology, recurrent tumors, lesions with ill-defined clinical margins, perineural or intravascular invasion, tumors arising in irradiated skin, and host immunosuppression. Other surgical options include simple excision, curettage, and cryosurgery.
Nonsurgical treatments for SCC may be used as a surgical adjuvant (decrease size of lesion before surgery or decrease recurrence rates after excision) or, in the case of superficial or in situ lesions, serve as the primary method of treatment. Current options for nonsurgical treatment include PDT, imiquimod, diclofenac, epidermal growth factor receptor (EGFR) inhibition (erlotinib, cetuximab), and external beam radiation.
PDT
PDT is a therapeutic option for SCC in situ lesions arising in patients who are poor surgical candidates or those who prefer noninvasive measures. Because of high recurrence rates and risk of metastatic disease, PDT is not a recommended treatment modality for invasive SCC tumors. A few case reports and series quote recurrence rates of 0% to 52% for SCC in situ and 82% for invasive SCC lesions.
Imiquimod
Current evidence supports the limited use of topical imiquimod 5% cream in the treatment of SCC in situ lesions occurring in low-risk individuals or patients who are poor surgical candidates. A randomized, double-blind, placebo-controlled study showed 73% (11 of 15) of biopsy-confirmed SCC in situ lesions achieved clearance after 16 weeks of therapy (applied once daily, 5 times a week). There were no documented cases of recurrence during a 9-month follow-up. Limitations to therapy include high rates of adverse effects (erythema, pruritus, and pain), lower clearance rates than other treatment modalities, and dependence on patient adherence to medication application.
Diclofenac
Diclofenac is a nonsteroidal antiinflammatory drug that works to inhibit the cyclooxygenase 2 enzyme, which is believed to be upregulated in NMSC lesions, thus promoting angiogenesis and impaired apoptosis. Topical diclofenac 3% gel has been approved only for the treatment of AKs; however, a few case series showed clearance of SCC in situ lesions with daily treatment of 4 to 12 weeks. One study showed no clinical evidence of SCC recurrence during a 10-month to 12-month follow-up period. Further study is required to determine the optimum dosing regimen of diclofenac 3% gel, because this remains largely unknown.
EGFR inhibition
The EGFR is an extracellular signaling receptor within the ErbB tyrosine kinase receptor family. The extracellular binding domain found on keratinocytes is a target of multiple different ligands, including epidermal growth factor, epiregulin, and transforming growth factor α. Activation of the EGFR receptor by these various ligands stimulates keratinocyte proliferation, leading to increased cell survival and resistance to apoptosis. Keratinocytes within SCC lesions often show overexpression of the EGFR, leading to unrestricted cell growth. Advanced SCC lesions contain EGFR mutations in approximately 43% to 73% of cases, which may lead to a more aggressive tumor phenotype.
Current treatment options for EGFR inhibition target either extracellular ligand binding inhibition or inactivation of intracellular tyrosine kinase signaling pathways. Cetuximab is a chimeric monoclonal antibody directed against the EGFR extracellular receptor domain and is FDA approved for the treatment of recurrent or metastatic SCC of the head and neck. A large study comparing weekly cetuximab infusions with radiotherapy with radiotherapy alone showed improved locoregional control and overall survival rate in the cetuximab treatment group. The second method of EGFR is accomplished by erlotinib and gefitinib, which disrupt the intracellular signaling pathway of the tyrosine kinase domain. In a small case study, gefitinib was reported to have an 11% response rate and 53% control rate in patients with head and neck SCC.
Radiotherapy
External beam radiation can function as both an adjunctive therapy to surgical excision or serve as primary treatment of head and neck SCC lesions in patients who are poor surgical candidates. Radiation may also play a role in palliative care for symptomatic relief of incurable skin cancers. Adjunctive treatment of complex SCC lesions with high-risk factors for recurrence (desmoplastic features, perineural invasion) leads to a decreased local recurrence and increased overall disease-free survival rates. One study of 167 patients with SCC and perineural invasion revealed a local recurrence rate of 43% with surgical excision alone compared with 20% with excision and adjunctive radiation. Likewise, disease-free survival increased from 53% to 73% in the 2 groups, respectively. Radiotherapy may be considered for cases in which tumor cells are close to surgical margins or in which negative margin control is not possible.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





