Non-Necrotizing Infections of the Dermis and Subcutaneous Fat: Cellulitis and Erysipelas: Introduction
|
Epidemiology
Skin and soft tissue infections (SSTI) are characterized by clinical findings that include an acute, tender, spreading, edematous, suppurative inflammation of the skin, subcutaneous fat, or muscle, often associated with systemic symptoms of malaise, fever, chills, and local pain. Cellulitis is an infection of the dermis and subcutaneous fat, while erysipelas is a more superficial variant affecting the superficial dermal lymphatics and adjacent tissues. Along with the pyodermas (see Chapter 176), cellulitis and erysipelas, are the predominant forms of non-necrotizing SSTI, which account for 7%–10% of hospitalizations in North America.1 Over the past two decades, the incidence of SSTI has increased faster than the incidence of other acute infections, paralleling the rise of methicillin-resistant Staphylococcus aureus (MRSA) rates.2
Cellulitis and erysipelas are usually caused by S. aureus or β-hemolytic Streptococci [primarily group A Streptococcus (GAS)] (see Table 178-1). Factors that increase the likelihood of SSTI include exposure to pathogenic organisms, local breach of the skin barrier function (including atopic dermatitis, and less often, allergic contact dermatitis, psoriasis, trauma, intravenous drug use, surgical and cosmetic procedures, toe-web intertrigo, arthropod bites, and chronic ulcers), immunocompromise (including acquired immunodeficiency syndrome [AIDS], diabetes, end-stage renal disease/dialysis, neutropenia, cancer, and immunosuppressive medications), obesity, and circulatory compromise (see Chapter 175).
Type of Infection | Most Common Cause (s) | Uncommon Causes |
| Erysipelas | Group A Streptococcus | Group B, C, and G Streptococci, Staphylococcus aureus |
| Cellulitis | S. aureus, group A Streptococcus | Group B, C, and G Streptococci; Streptococcus iniae; Pneumococcus; Haemophilus influenzae (children); Escherichia coli; Proteus, other Enterobacteriaceae; Campylobacter jejuni; Moraxella; Cryptococcus neoformans; Legionella pneumophila, Legionella micdadei; Bacillus anthracis (anthrax); Aeromonas hydrophila; Erysipelothrix rhusiopathiae; Vibrio vulnificus, Vibrio alginolyticus, Vibrio cholera non-01 |
| Cellulitis in children | S. aureus, group A Streptococcus | Group B Streptococcus (neonates) |
| Facial/periorbital cellulitis | S. aureus, group A Streptococcus | Neisseria meningitides, H. influenzae (young children) |
| Perianal cellulitis | Group A Streptococcus | S. aureus |
| Cellulitis secondary to bacteremia | Pseudomonas aeruginosa | V. vulnificus; Streptococcus pneumoniae; group A, B, C and G Streptococci |
| Cellulitis associated with water exposure | E. rhusiopathiae (erysipeloid) | V. vulnificus, A. hydrophila, Mycobacterium marinum (nodular lymphangitis), Mycobacteria fortuitum complex |
Several risk factors, relatively specific to erysipelas, include extremes of age (children and the elderly), diabetes mellitus, and nephrotic syndrome.3 In the adult patient with erysipelas, lymphedema (including congenital lymphedema), venous stasis, web intertrigo, and obesity are common risk factors. Lymphedema in particular may be an important feature in erysipelas even when subclinical and only evident by lymphosyntigraphy.4
For all variants of cellulitis, there is less predictability in immunocompromised hosts where organisms may include a range of traditional and rare pathogens, usual commensals, yeast, fungi, and parasites. Because traditional patterns of symptoms and physical findings may be lacking or nonspecific in immunocompromised patients, determining an etiologic diagnosis is especially important.5 In both immunocompetent and immunocompromised hosts, bullae, necrosis, or gas-forming bacterial infections signal urgency in establishing the location of infection and in defining the cause (see Chapter 179).
Etiology, Microbiology, and Pathogenesis
Pathogens isolated from cellulitis are split primarily between S. aureus and GAS; the best estimates place the rate of S. aureus cellulitis at approximately 50%, and GAS at approximately 35%.6 However, microbiologic studies are limited by the low yield of cultures from cellulitis (see Section “Diagnostic Studies”), and anecdotal experience suggests that S. aureus may now be a far more common cause of cellulitis than GAS. Haemophilus influenzae is an important cause of cellulitis in much of the world, but the widespread use of the Haemophilus influenza type b vaccine has greatly decreased the incidence in the United States.
The remainder of cellulitis cases are caused primarily by groups B and G Streptococcus,7,8 enteric-Gram-negative rods,9 coagulase-negative Staphylococcus,10 and Streptococcus pneumoniae.11 These unusual pathogens are particularly common and problematic in association with extremes of age, prolonged hospitalization, percutaneous intravascular lines, diabetes, obesity, immunocompromised states, and glucocorticoids.8 Liposuction and percutaneous drug use are reported risk factors that may also lead to cellulitis from less common organisms.
Erysipelas is a distinct type of superficial cutaneous cellulitis with marked dermal lymphatic vessel involvement. GAS has classically been considered the predominant cause of erysipelas, but erysipelas may also be caused by S. aureus and group C or G Streptococcus.
Both S. aureus and GAS possess effective mechanisms of bypassing the immune system and establishing infection. GAS appears capable of inactivating cathelicidin LL-37, thereby mediating resistance to the innate immune system.12 Although it is classically regarded as an extracellular pathogen, GAS also appears able to evade immune detection and antibiotic therapy by entering macrophages and endothelial cells.13,14 Both GAS and S. aureus may produce exotoxins that result in systemic toxic reactions, including toxic shock syndrome (see Chapter 177). Panton–Valentine Leukocidin (PVL) is a β-pore forming toxin produced by many strains of S. aureus that damages leukocytes and predisposes to severe SSTI and other infections.15
Antibiotic resistance is of increasing concern, particularly in the case of MRSA, which, in many series across disparate geographic areas in the United States and Europe, now accounts for more than 50% of cellulitis from which a pathogen could be cultured.16–19 Healthy individuals are frequently affected, but certain populations appear to be at particular risk for MRSA skin infections, including those with immunosuppression, chronic illness (including end-stage renal disease), prisoners,17 athletes,20 and men who have sex with men.21 Other risks noted in the literature include youth, recent sexual contact, presence of abscess, low body mass index, spontaneity of infection, and group home living.16
Methicillin resistance arises from the mecA gene, a component of a mobile genetic element, the staphylococcal cassette chromosome (SCCmec). This gene results in the production of an altered penicillin-binding protein (PBP2a), with a lower affinity for β-lactam groups, resulting in a minimum inhibitory concentration (MIC) to oxacillin of at least 4 μg.22 This mechanism imparts resistance to all β-lactam antibiotics currently available, including penicillins, cephalosporins, monobactams, and carbapenems. Similar resistance mechanisms to β-lactams have been increasing in several important pathogenic strains of coagulase-negative Staphylococcus species, including S. sciuri and S. haemolyticus.23,24
Relative to infection with Methicillin-sensitive S. aureus (MSSA) and other pathogens, infection with MRSA is associated with a higher rate of adverse outcome and treatment failure,25 as well as higher economic costs of care.26 These unfavorable outcomes likely arise from the virulence factors closely associated with dominant MRSA strains in both community and nosocomial settings. Many MRSA strains, including the CA300 strain, which has emerged as the dominant community-acquired (CA) strain in the United States, have a very high rate of PVL expression,27,28 and they also appear to be more effective colonizers than MSSA strains.29
Both hospital-acquired (HA) and CA-MRSA strains have accumulated mutations that result in resistance to other classes of antibiotics, including macrolides, tetracyclines, fluoroquinolones, and sulfonamides, though the resistance patterns to these non-β-lactam antibiotics vary widely by region. With increased use of mupirocin and chlorhexidine as adjunctive therapies for MRSA, or as part of decolonization regimens, resistance to these topical agents is also on the rise, topping 10% in some settings.30,31 Resistance to mupirocin has been associated with increased resistance to other antibiotics,30 and with poor outcome measures, including increased mortality in at least one study of MRSA in an intensive care unit setting.32 In addition, resistance to the drugs often considered “salvage” medications, including vancomycin19 and linezolid,33 is of increasing prevalence and concern.
Vancomycin-resistant Enterococcus is most often a colonizing organism in SSTI, but it may act as a pathogen in nosocomial SSTI in immunocompromised patients.34 Despite its relatively low virulence, VRE is of increasing concern because of its ability to transmit resistance to other bacterial species, and because of the increasing prevalence of immunocompromised patients worldwide from HIV disease/AIDS and immunosuppressive medications.35
Skin Signs and Symptoms
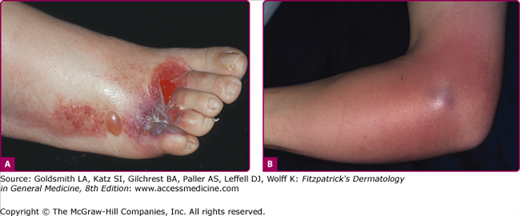
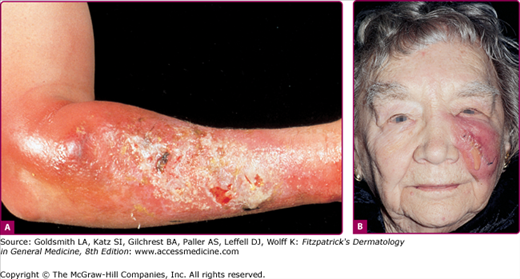
Cellulitis presents with erythema, pain, firm and tender induration, and less commonly, fluctuance. The erythema may rapidly intensify and spread. The margins are generally indistinct. In some cases of cellulitis, the overlying epidermis undergoes bulla formation or necrosis, resulting in extensive areas of epidermal sloughing and superficial erosion (Figs. 178-1 and 178-2). Regional lymphadenopathy may be associated with cellulitis on an extremity. In older individuals, thrombophlebitis may complicate lower leg cellulitis. Systemic symptoms, such as fever, chills, and malaise are variable, and sometimes may antedate localizing complaints and signs of SSTI. In a study of 50 patients with cellulitis, only 26% had fever higher than 38°C (100.4°F). A potential portal of entry was identified in 66% of patients.36
The infection may also cause deeper necrosis, resulting in dermal and subcutaneous abscess formation, fasciitis, and myonecrosis. Pain in the absence of erythema, or out of proportion to the appearance of the local area, should raise suspicion for an early deep-seated infection. Crepitus is a rare sign that signifies a gas-forming pathogen (see Chapter 179).
Cellulitis usually presents at the site of an antecedent lesion, including acute and chronic ulcers, traumatic wounds (abrasions, lacerations, animal and human bites), surgical procedure sites, dermatoses, or percutaneous catheters. Less commonly, bacteremia from systemic infections such as osteomyelitis and diverticular abscesses may cause SSTI.
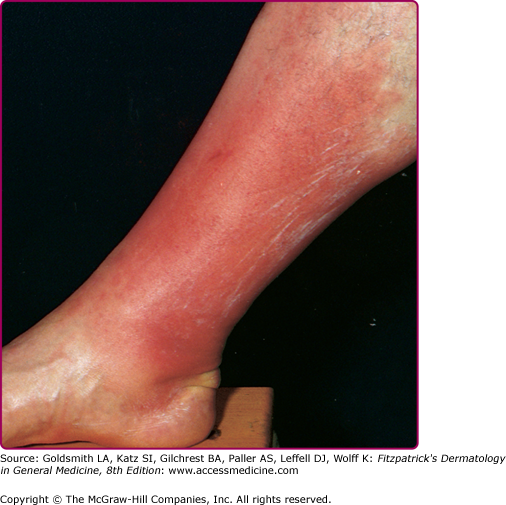
Although erysipelas shares many clinical features with classical cellulitis (pain, tenderness, erythema, and edema), the plaque-like edema has a more sharply defined margin to normal tissue, and the erythema is classically bright red (Fig. 178-3). The surface findings are often described as peau d’orange (skin of an orange) in appearance. In the presence of antecedent edema or other anatomic abnormalities, the margin between normal and diseased soft tissue may be more obscure, much as in primary cellulitis.
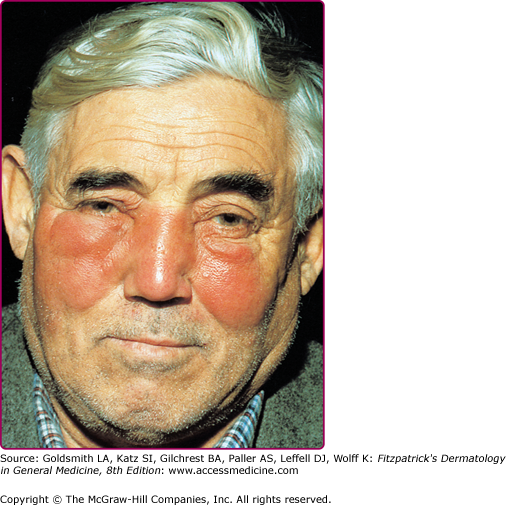
Seventy-five to 90% of cases involve the lower extremities, while the face is affected in 2.5%–10% of cases.37 Facial erysipelas begins unilaterally but may spread by contiguity over the nasal prominence to involve the face symmetrically (Fig. 178-4). There may not be an obvious portal of entry, and skipped areas may confuse the nature of the process. The oropharynx is a common portal of entry, and throat culture may show GAS. Inflammatory edema can extend to the eyelids, but orbital complications are rare.
Fever may precede local signs, and occasionally before distal extremity findings, patients complain of groin pain caused by swelling of femoral lymph nodes. Lymphangitis and abscess are not common, but the process may spread rapidly from the initial lesion. Infrequently, bullae or epidermal sloughing may occur in the involved area.38
Surgical wound infections are the most common adverse events in hospitalized patients undergoing surgery and are classified as incisional (superficial) or deep.39 Incisional wound infections involve the skin, subcutaneous tissue, and/or muscle (see Fig. 178-2B). Up to 80% of wound infections are incisional. A wound is considered to be infected if there is drainage of purulent material and evidence of inflammation. Incisional infections present with erythema, pain, tenderness, local swelling and often, with low-grade fever. Cultures of the purulent drainage most often grow S. aureus. However, the microbiologic profile of cellulitis complicating surgery may reflect the structures incised during the procedure, such as an Enterobacteriaceae cellulitis after a genitourinary operation.40
Deep infections involve structures adjacent to the surgical wound that were entered or exposed during the procedure, such as subfascial layers, viscera, and/or spaces within the peritoneum, thorax, or joints. Deep-wound infections may be more subtle and delayed, and often present as fever of uncertain cause. Progressive bacterial synergistic gangrene and other variants of gangrenous cellulitis can arise in surgical wounds or around sutures (see Chapter 179).
The complications of superficial and deep-wound infections include poor healing, bacteremia, local and systemic effects of prolonged hospitalization, poor nutrition, residual compromised tissue integrity, and the consequences of prolonged antibiotic therapy. Additionally, wounds infected with toxin-producing S. aureus or GAS may result in systemic complications such as toxic shock syndrome or scarlet fever (see Chapter 177).
Pressure ulcers, particularly those located in the sacrum of elderly, frail, malnourished individuals, become contaminated by a variety of facultative and anaerobic microorganisms from the skin and the bowel, including S. aureus, Enterococci, Pseudomonas aeruginosa, and Bacteroides fragilis. In addition to pain and cellulitis, the ulcer may eventually be complicated by bacteremia, often polymicrobial, or the underlying bone can become infected. Given a high burden of colonizing organisms, the identity of the active pathogen(s) may be difficult to determine. Pressure ulcers complicated by cellulitis or sepsis should be urgently debrided.41
Domestic dog and cat bites are common and can give rise to cellulitis caused by Pasteurella multocida, Capnocytophaga canimorsus (especially in asplenic individuals), and a host of other aerobes and anaerobes from the animal’s mouth or the skin of the infected individual. Dog bites are often accompanied by a crush injury that devitalizes tissues. The bite of cats can inject organisms (via sharp incisors) deep into tissues, including joint spaces, tendon sheaths, or bone (see Chapters 180 and 209).
With rates of cellulitis approaching 15%, human bites have a higher incidence of infection than do animal bites because of the mix of oral bacteria (aerobes and anaerobes), as well as the crush injury imparted by the bite.42,43 Organisms include various Streptococci, S. aureus, Eikenella, Corynebacterium, and the anaerobic Peptostreptococci and Peptococci. Cellulitis from bites should be monitored carefully for progression to a necrotizing infection.