Nipple-Sparing Mastectomy and Reconstruction: Indications, Techniques, and Outcomes
Scott L. Spear
Shawna C. Willey
Catherine M. Hannan
Costanza Cocilovo
Introduction
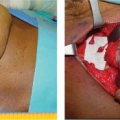
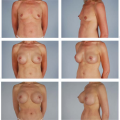
Nipple-sparing mastectomy (NSM), or subcutaneous mastectomy, is the ultimate form of skin-sparing mastectomy in that it includes preservation of the nipple pending pathologic assessment of the subjacent tissue. Immediate reconstruction after nipple-sparing mastectomy may allow for better cosmetic results with lower risk of complications as compared to reconstruction after total mastectomy. However, the oncologic safety of this procedure remains the subject of study.
Subcutaneous mastectomy for primary breast cancer or risk reduction has been described for several decades. In 1962, Freeman (1) pioneered this surgical procedure, but it was eventually discredited because of unclear selection criteria, poor cosmetic results, high rate of complications, and lingering questions about its oncologic safety or efficacy (2). For most of the 20th century, removal of the nipple-areolar complex was a standard part of a mastectomy, despite the fact that the nipple is a relatively uncommon site for breast cancer to develop (3). The most often observed primary neoplasia of the nipple is Paget’s disease of the breast (intraepidermal tumor cells of the nipple), which remains an uncommon presentation of breast malignancy, accounting for 1% to 3% of all breast tumors (4). According to the largest population-based reference resource for breast cancer in the United States—the Surveillance, Epidemiology, and End Results (SEER) registry of the National Cancer Institute—of 157,546 women with invasive breast cancer between 1973 and 1987, there were 1,763 women (1.1%) who had histologically confirmed Paget disease (3). That early report suggested that the incidence of Paget disease has been rising in the United States, although those findings were based on SEER data from the years 1973 to 1987. Paget’s disease may occur in the nipple in conjunction with an invasive cancer mass, with underlying ductal carcinoma in situ (DCIS), or alone without any underlying invasive breast carcinoma or DCIS. The associated underlying cancer may be located centrally in the breast adjacent to the nipple, or it may be located peripherally in the breast. Early reports described Paget’s disease alone without an underlying cancer as rare, representing at most 8% of those few patients with Paget’s disease. Fortunately, therefore, carcinoma of the nipple is extremely rare. Meanwhile, with the advent of screening protocols allowing for earlier breast cancer detection and therefore smaller tumors and lower-stage cancers, it has become increasingly tempting to preserve more and more of the native breast skin, diverging from the well-accepted concept of “skin-sparing mastectomy” to total skin envelope preservation.
With ever-increasing expectations of improved cosmetic results from breast reconstruction, it would only seem natural that nipple-sparing mastectomy would become the next consideration. It has the potential to remove virtually all glandular tissue, similar to other mastectomies, yet preserve the nipple-areola complex, as is done with breast-conserving treatments for breast cancer. It needs to be emphasized, however, that regardless of mastectomy technique, including modified radical mastectomy, it is unlikely to completely remove 100% of breast tissue.
If the use of nipple-sparing mastectomy is questionable because of the possibility of an increased risk of local recurrence in the nipple area, this procedure may be more acceptable in the prophylactic setting. In that setting, nipple-sparing mastectomy has been shown to dramatically reduce the incidence of breast cancer in high-risk patients (5).
Indications
Risk Reduction Mastectomy
The concept of nipple-sparing mastectomy was first popularized in the 1960s and 1970s as subcutaneous mastectomy (1). That procedure for risk reduction quickly fell out of favor for a number of good reasons. Most significantly, there were no evidence-based selection criteria and therefore no possibility of ever demonstrating efficacy. Beyond that, the reconstructive techniques of the day were relatively crude, the results were inconsistent, the best outcomes were not impressive, the complication rate was high, and a significant amount of breast tissue was often intentionally left behind.
All this changed with the seminal report by Hartmann et al. (6,7) published in the New England Journal of Medicine in 1999. Over the preceding 20 years or more, the Mayo Clinic had been a center of sorts for prophylactic mastectomy, largely of the historic subcutaneous mastectomy variety. The retrospective data from that series of 639 women demonstrated that prophylactic mastectomy did indeed have a protective benefit, reducing the risk of breast cancer in both high-risk and moderate-risk groups by 81% and 94%, respectively. Ninety percent of the mastectomies in that series were nipple-sparing. Breast cancer developed in 7 women after prophylactic mastectomy. Six cancers were confined to the chest wall at diagnosis and were specifically not in the area of the nipple-areola complex. One patient in the high-risk group presented with bone marrow metastases from adenocarcinoma with no evidence of breast disease. There was no statistically significant difference in the cancer-preventing benefit whether the nipple was removed or retained (8).
In the context of risk reduction, other series report similar experience. McDonnell et al. (9), also from the Mayo Clinic, reported in 2001 in the Journal of Clinical Oncology on a series of 745 women with a first breast cancer and a strong family history of breast cancer who underwent unilateral prophylactic
mastectomy of the opposite breast between 1960 and 1993, resulting in a projected 94% to 96% percent risk reduction. All told, there were 8 breast cancers versus 156 predicted. Forty-one percent of the mastectomies spared the nipple, while 59% did not. The 8 cancers that developed were evenly split, 4 and 4, between the nipple-sparing and nipple removal groups. None of the 8 cancers that did occur developed near the nipple.
mastectomy of the opposite breast between 1960 and 1993, resulting in a projected 94% to 96% percent risk reduction. All told, there were 8 breast cancers versus 156 predicted. Forty-one percent of the mastectomies spared the nipple, while 59% did not. The 8 cancers that developed were evenly split, 4 and 4, between the nipple-sparing and nipple removal groups. None of the 8 cancers that did occur developed near the nipple.
In 2004, Crowe (10) reported on 17 nipple-sparing prophylactic mastectomies performed from 2001 to 2003. Crowe recommend a lateral incision for improved nipple-areola survivability. There were no occult cancers seen on frozen or permanent examination beneath the nipple, and no cancers had developed since.
In 2006, Sacchini (2) published in the Journal of the American College of Surgeons a larger multicenter experience including the Memorial Sloan-Kettering Cancer Center in New York and major cancer centers in Sao Paulo, Brazil, and Milan and Padua, Italy. All together 55 patients underwent nipple-sparing prophylactic mastectomies. There were no recurrent or new cancers in the nipple with mean follow-up of 24 months. Two cancers did develop after prophylactic mastectomy, 1 in the axillary tail 24 months postoperatively and 1 in the upper-outer quadrant at 62 months. The majority of their nipple-sparing procedures were performed via a periareolar incision, which included coring out the nipple. Twenty-two of 192 nipples (including therapeutic mastectomy in their series) suffered some degree of necrosis, 9 of which resulted in loss of more than one third of the nipple-areola complex.
Rebbeck (11) et al. published a combined multicenter experience from the multicenter Prose study group of 483 BRCA1 and 2–positive women, including 105 who underwent bilateral prophylactic mastectomy, including 29 nipple-sparing procedures. Two of those 105 developed breast cancer, in contrast to 184 cancers among 378 patients in the control group, yielding a 90% or more risk reduction. The 2 cancers that developed included 1 in the axilla and 1 in the breast. There was no statistically significant difference in the occurrence of cancer between the nipple-sparing and non–nipple-sparing groups.
Nipple-Sparing Mastectomy in the Treatment of Breast Cancer
If surgeons have been reluctant to embrace NSM in the context of risk reduction, then they have been even more reluctant to do so in the face of diagnosed breast cancer. There have been many inconsistent reports from the last 20 to 30 years regarding the likelihood of nipple involvement with cancer in the face of known ipsilateral breast cancer. Reports of nipple involvement with cancer varied widely from 0% to 58% (2). Much of those data came from reports in the 1970s and 1980s based on examination of the mastectomy specimens from an era of later diagnosis and more advanced disease. Furthermore, the methodology for tissue examination of those studies and the criteria for describing “involvement” were not uniform.
With the increased interest in skin-sparing and even nipple-sparing mastectomy, more-relevant studies have recently been done. Laronga et al. (12) from the MD Anderson Cancer Center in Houston, Texas, reported in 1999 on 326 patients whose breast specimen were examined after skin-sparing mastectomy. They described how earlier studies correlated the risk of occult nipple-areola complex (NAC) involvement was greater when the primary tumor was close to the NAC, larger than 2 cm, poorly differentiated, and associated with positive axillary lymph nodes. They found 16 (5.6%) instances of occult tumor involvement in the examined specimens. They believed that 4 of these would have been identified on frozen section if it had been performed. The only statistically significant predictors of nipple involvement were location (subareolar or multicentric) and axillary nodal status. The authors speculated that their finding of a relatively low incidence of occult NAC involvement might reflect their selective preoperative criteria for skin-sparing mastectomy in the first place. They concluded that it would be appropriate to offer NAC preservation in axillary node negative patients with tumors located on the periphery of the breast. They estimated that in that group, the probability of missing occult tumor in the NAC would be less than 2%.
Jensen (13) in 2002 defended preservation of the nipple-areola complex in DCIS, depending on the ability to get surgical margins of at least 10 mm. Citing Silverstein et al. (14), who showed that when DCIS is removed with a 10-mm margin the chance of recurrence in the preserved breast is the same with our without additional radiation, he argued that the chance of recurrence in this setting is approximately 2% regardless of the size or pathologic characteristics of the DCIS. Although these data were from lumpectomy (breast-conserving) cases, Jensen suggested that this can be extrapolated to mastectomy patients as long as a frozen section or permanent pathologic section shows 10 mm of breast tissue separating the nipple-areola complex from the underlying breast parenchyma. If this pathologic margin is clear, then he believed it was reasonable to leave the nipple-areola complex intact.
Jensen went on to reason that if one carefully analyzes the data from the National Surgical Adjuvant Breast and Bowel Project Protocol B-06 (15,16,17), the definitive study on the treatment of stage 1 and 2 breast cancer, preservation of the nipple and/or areola can be extended to carefully selected patients with invasive breast cancer. In that study, women with negative or positive axillary lymph nodes and tumors less that 4 cm in diameter were randomly assigned to one of three treatment protocols: mastectomy, lumpectomy alone, or lumpectomy with radiation. An important criterion of the B-06 protocol was clear surgical margins, defined as not having tumor present at the cut margin (a single cell width was regarded as a clear margin). In two of three cohorts (lumpectomy alone and lumpectomy with radiation), the nipple-areola complex was preserved. At 12 years’ follow-up, the cumulative incidence of a recurrence of tumor in the ipsilateral breast was 35% in the group treated with lumpectomy alone and 10% in the group treated with lumpectomy and breast irradiation (p < 0.001). However, no statistically significant difference in survival was observed. Even at 20 years of follow-up, no difference was seen between mastectomy compared to lumpectomy with or without radiation in terms of disease-free survival (p = 0.26), distant disease-free survival (p = 0.34), or overall survival (p = 0.57). Jensen therefore argued that there is no obvious survival advantage in removing the nipple-areola complex when surgical margins are clear. He further hypothesized that if at least 1 cm of breast tissue beneath the nipple-areola complex is free of tumor, then in carefully screened patients meeting appropriate selection criteria, it is reasonable to retain the nipple. Patients need to be counseled that based on this large data set, retaining the nipple in the setting of cancer can lead to an increased risk of recurrence, but that it may not translate into lower survival rates.
Table 23.1 Summary of Therapeutic Nipple-Sparing Mastectomy Literature | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree