Nipple-Sparing Mastectomy
G. Patrick Maxwell
Pat Whitworth
Allen Gabriel
Introduction
During the last century, breast reconstruction has evolved from a rarely performed surgical venture to a daily occurrence that has become an important part of the rehabilitation process following mastectomy or lumpectomy. The aesthetic quality of the reconstructions fostered by technical advances has emerged from that of the amorphous blobs appearing as breast mounds to nearly normal appearing breasts. Symmetry, which was hardly possible and seldom achieved, is now the standard for which we strive. Along the same line the surgical management of breast cancer has undergone an evolution from more radical mastectomies to less invasive breast conservation therapies and now to nipple-sparing mastectomies (NSMs). NSM is a procedure that combines skin-sparing mastectomy with preservation of the nipple-areolar complex (NAC). Several studies are emerging to attest to the efficacy and safety of the procedure.
One may ask, why consider saving the nipple since we have advanced reconstructive techniques that can achieve similar goals? Nipple-areola reconstruction has always represented the final stage of breast reconstruction, whereby a reconstructed breast mound is transformed into a breast with maximal realism when compared with the patient’s opposite breast. Essentially all postmastectomy patients are fraught with distress brought on by the diagnosis of breast cancer and suffer a severe alteration of body image and the resultant adverse psychological consequences (1). Preservation of the nipple-areola complex can be an extremely vital part of their rehabilitation in the sense that it visually transforms the mastectomy skin into a breast immediately following surgery. As surgeons we strive to achieve the most aesthetic breast form, and the preservation of NAC, if possible, adds to our goals. Nipple-sparing mastectomy was attempted in the 1980s but never gained popularity due to the controversies surrounding oncologic safety (2). Now better technologies for improved preoperative staging and assessment of lesion distance from NAC are creating support for the return of this concept.
History
The classic subcutaneous mastectomy sparing the NAC was historically reported by Freeman in the 1960s. He performed the procedure in the presence of benign breast lesions but did not document it for breast cancer treatment or as a risk-reduction procedure (3,4).
In recent years, there has been a sudden increase in reports of NSM series for prophylaxis or cancer treatment, evidencing renewed interest in this technique. Of an approximate total of 1,868 NSMs performed for breast cancer treatment published in the recent literature (5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27), only 3 local recurrences within the NAC have been reported (10,11,13,16), representing a proportion of local events of 0.16% attributed to NAC preservation. It is noteworthy that most of these studies have a short follow-up, thus rendering definite conclusions based on this information premature.
Nipple-Areolar Anatomy
The use of NSM for both breast cancer treatment and risk reduction is increasing. There are no randomized data comparing nipple-sparing mastectomy with standard mastectomy techniques. Therefore, detailed information about NAC microanatomy is essential for understanding the issues faced when considering its preservation.
There is evidence to suggest that ductal and lobular breast cancer arises in the terminal duct/lobular unit (TDLU) (28). Stolier et al. examined 32 nipples and concluded that all TDLUs were found at the base of the nipple, with none located near the tip (28). Others have also shown that majority of breast tumors originate within the TDLU (29,30,31). This information is useful when discussing nipple-sparing mastectomy for risk reduction surgery, including that for women with BRCA1/2 mutations.
There is variation in the number of ducts reported, and little was known about the spatial location of ducts, their size, and their relationship to orifices on the surface until Rusby et al. reported the findings of nipple specimens from 129 consecutive mastectomies. They showed that many ducts share a few common openings onto the surface of the nipple, explaining the observed discrepancy between number of ducts and number of orifices. Neither duct diameter nor position predicted whether a duct system will terminate close to the nipple or pass deeper into the breast (32). There was also concern about the viability of the nipple tip following coring out the ducts. Rusby et al. presented their findings after exploring the precise anatomic relationship between ducts and vasculature within the nipple. Their study investigated nipple microvessels and their position relative to ducts and concluded that ducts can be excised leaving a rim of nipple tissue that contains a large proportion of microvessels (33). Finally, the most important study published by the same group noted the detailed support and understanding that was needed for surgeons to perform these procedures. The authors built a predictive model using preoperative information to aid in the selection for NSM. This clinical tool included tumor size and distance from the nipple to help improve candidacy and appropriate patient selection for nipple-sparing mastectomy (34).
Rusby’s series of ex vivo procedures provided information that can be used to modify surgical and pathologic techniques for nipple-sparing mastectomy (35). The precise identification of the duct margin directly beneath the nipple proves to be difficult once the duct bundle has been divided. In their series, successful retroareolar margin identification was achieved by grasping the duct bundle with atraumatic forceps as soon as it
became exposed. A cut made below and above the forceps resulted in a full cross section of the duct bundle. Modification of technique resulted in more complete excision of duct tissue (35).
became exposed. A cut made below and above the forceps resulted in a full cross section of the duct bundle. Modification of technique resulted in more complete excision of duct tissue (35).
This detailed information about NAC microanatomy is essential for understanding what is faced when considering its preservation.
Risk Reduction Mastectomy
The management of women at high risk for breast cancer presents a clinical dilemma to the health care provider as well as to the woman. Current options include surveillance, prophylactic surgery (mastectomy and/or oophorectomy), and/or chemoprevention (36). These patients can be divided into three groups: patients with (1) positive BRAC1/BRCA2, (2) personal or family history of cancer following unilateral mastectomy for cancer, and (3) severe fibrocystic disease with strong family history of cancer.
Hartmann et al. have showed that prophylactic mastectomy is associated with a substantial reduction in the incidence of subsequent breast cancer not only in women identified as being at high risk on the basis of a family history of breast cancer but also in known BRCA1 or BRCA2 mutation carriers (37). McDonnell et al. estimated the efficacy of contralateral prophylactic mastectomy in 745 women with a personal and family history of breast cancer between 1960 and 1993. Of the 745 women, 388 were premenopausal (age <50 years) and 357 were postmenopausal. The authors concluded that the incidence of contralateral breast cancer seems to be reduced significantly after contralateral prophylactic mastectomy in women with a personal and family history of breast cancer (38).
In the prophylactic setting, a large study by Hartmann et al. (39) showed that the risk of developing a primary inva-sive tumor within the NAC after subcutaneous mastectomy in moderate- to high-risk patients is outstandingly low. Subcutaneous mastectomy and NSM were compared, and there was no significant difference in the incidence of breast cancer between these two procedures.
In the setting of prophylactic mastectomy, NSM can be considered virtually in all cases after ruling out malignancy and discussing with patients all risk-reducing strategies. Preoperative evaluation for NSM should include complete imaging studies, preferentially breast magnetic resonance imaging (MRI).
Mastectomy for the Treatment of Cancer
Nipple-sparing mastectomy has been evolving over many years and it is crucial for the plastic surgeon and the oncologic surgeon to screen patients and select the best candidates for this operation. The goal, as with any case of breast cancer, is to foremost treat the breast cancer and perform the oncologically safest surgery followed by reconstruction. If a patient is a poor candidate for breast conservation therapy (BCT), this patient may also be a poor candidate for NSM. Nipple-sparing mastectomy is not meant to replace BCT; however, it serves as an option for patients who may not desire to undergo radiation therapy or who may have small amount of breast tissue such that a lumpectomy alone would remove more than 50% of the breast tissue.
Screening patients is as crucial as in any other reconstructive case, and patients should be appropriate candidates. The incision choice, whether lateral or vertical breast, inframammary fold, former biopsy site, or reduction pattern, should never compromise the efficacy of the oncologic resection (Fig. 24.1). All incisions should be an option, and the best should be chosen to achieve maximal safe resection while keeping the aesthetic goals in mind.
Selection criteria for NSM can be proposed and refined with ongoing experience, and keeping in mind that indications for this procedure require a balance between clinical stage and tumor biology, selection could be proposed for patients with breast cancer that meet the following minimum criteria (27,34):
No clinical involvement of the NAC (including nipple discharge)
Tumor size up to 3 cm (pending nodal status)
Tumor-to-nipple distance ≥2 cm
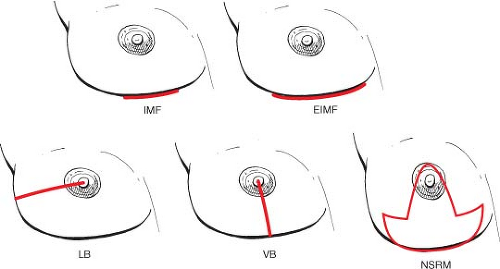 Figure 24.1. Incision options. EIMF, extrainframammary fold; IMF, intramammary fold; LB, lateral breast; NSRM, nipple-sparing reduction mastectomy; VB, vertical breast. |
Additional criteria should be used to evaluate candidacy of NSM such as patients with any skin involvement, inflammatory breast cancer, or multicentric disease should not undergo this procedure. Our goal is to achieve longevity with high quality of life; therefore superior oncological management should always be the primary treatment. One can argue that these maybe slight more conservative than other published criteria. Although positive axillary status has not been consistently correlated with risk of nipple involvement, this procedure should be discouraged in patients with extensive axillary node metastasis. In addition, most candidates for breast-conserving surgery who still prefer to undergo mastectomy could be evaluated for NSM (27).
It is worth reviewing the risk factors for lymph node involvement in order to achieve the appropriate candidates for NSM. The axillary lymph nodes (LNs) receive 85% of the lymphatic drainage from all quadrants of the breast; the remainder drains to the internal mammary (IM) chain. The likelihood of axillary LN involvement is related to tumor size and location, histologic grade, and the presence of lymphatic invasion (40,41).
Tumor Size and Margins
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








