Fig. 2.1
Botulinum toxin protein
Thus, Allergan, Inc. (Irvine, CA) must credit this physician-lead curiosity and clinical initiative with taking a little known orphan drug, Botox™ to what is now known as Botox Cosmetic™ (roughly $25 million to over one billion in annual sales). More importantly, the use of this molecule in aesthetic and reconstructive surgery has changed the contemporary approach to patients in complimentary fields.
The Botulinum toxins are naturally occurring complex proteins (heavy and light chain dimers) that in nature are surrounded by “inactive” complexing proteins. It should be noted that research presented at Toxins 2012 (Miami, FL, 2012) shows activity in membrane penetration and/or permeability related to these complexing proteins and their function is, at best, poorly understood. Such properties may explain subtle differences in clinical characteristics of the various BoNTA products. The “active” heavy and light chains are responsible for neuronal terminal end uptake and cleavage of proteins responsible for acetylcholine vesicle release into the neurosynaptic cleft, respectively [3].
Terminology in medicine is important and heretofore the protein basic science community has referred to the Botulinum toxins simply as “toxins” and has developed an industry standard abbreviation, by consensus, of BoNTA and BoNTB for the commercially available A and B serotypes of Botulinum toxins, respectively. While terms like “toxin” work for basic science community, referring to these medications (or any medications for that matter) in discussions with patients would be unnecessarily alarming and, in principle, inaccurate.
In practice all medications are “toxins” in large or incorrect dosages. This has led many clinicians to adopt (appropriately in the author’s opinion) alternative terminologies when describing this class of medications. Ideally, industry or a representative group of clinician thought leaders would meet and agree upon an appropriate standard clinical nomenclature. For now it appears the term “neuromodulator” seems to have gained acceptance. As defined by Mosby’s Medical Dictionary a neuromodulator is “a substance that alters nerve impulse transmission” [4]. While certain industry and clinician spokespersons have claimed to have “invented” the term neuromodulator, it is hardly a new term and such claims have generated controversy around the adoption of this term. For purposes of this chapter we will refer to the clinically available forms of Botulinum neurotoxin BoNT as neuromodulators (NMs).
Introduction of New NMs and Relevant Dosages
While Botox Cosmetic™ has become a mainstay workhorse and household name other relevant competitors have been introduced with varying success.
Myobloc™ (US WorldMeds, S. San Francisco, CA) the only B serotype neuromodulator is supplied in a sterile slightly acidic and stable solution ready for injection. Its efficacy and use in neurology was not found in clinical studies to be effective for aesthetic applications due to its shorter clinical effect and pain on injection [5, 6].
Effectively the US market has currently three available forms of BoNTA and one under FDA consideration.
Each of these products is provided in a dry form with instructions for reconstitution in sterile saline. Many clinicians prefer the use of preserved saline with reports of decreased pain on injection. The author uses normal saline. With slightly greater area of effect with Dysport™ (Medicis Corp Scottsdale, AZ), we use smaller volumes of diluent (1.5 ml) than with Botox (2.0 ml). Reported ranges in clinical use for diluent volume are generally accepted to be between 1.0 and 4.0 ml. The lower end volumes require very small and incremental injections and may be difficult to effectively cover larger muscle groups and the higher end volumes result in more local swelling and potentially diffusion-related side effects. This has not been proven to our knowledge in controlled trials, however, and it is advised that the clinician uses the mid range of diluent volumes.
Since its cosmetic indication market clearance in 2002 (and before) Botox Cosmetic™ remains the industry standard and its applications have expanded well beyond the early upper face indications [7, 8]. It is supplied as a lyophilized powder and the company has gone to great lengths to reduce the worldwide problem with forgery and false product distribution (often over the Internet) with the use of holograms and Botox Cosmetic™ product-specific identifiers.
Consistency in potency and clinical results as well as its established trade name are strong benefits of Botox Cosmetic™. The points of injection and dosages employed by the author are shown in Fig. 2.2.
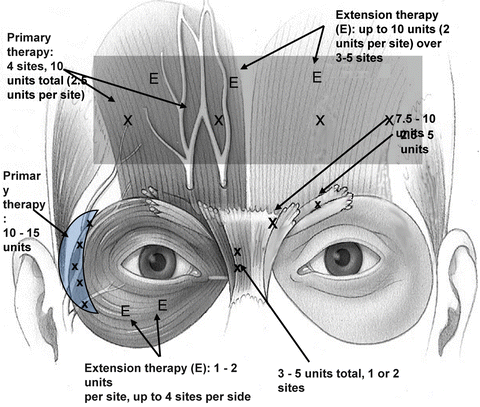

Fig. 2.2
BoTox cosmetic injection sites and dosages
Dysport™ was FDA-cleared in 2009 with a slow adoption curve largely due to overdosage in the frontalis muscle and resultant brow ptosis. This product has proven to be an excellent competitor for Botox Cosmetic™ and its wider area of effect can be harnessed to provide more effective rhytid effacement in the flatter orbicularis and frontalis muscle groups. A split face double blind study comparing Botox Cosmetic™ and Dysport for lateral orbital rhytids at 10 and 30 units, respectively, showed a 2:1 (61 of 90 patients) preferred the Dysport™ treatment side with a 17 % greater improvement over Botox Cosmetic™ at 1 and 3 months post-treatment [9]. The author’s treatment points and dosages are shown in Fig. 2.3.
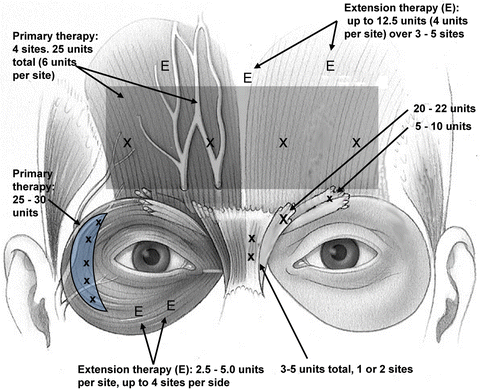

Fig. 2.3
DYSPORT injection sites and dosage
While individual products cannot be directly compared on a unit for unit basis most experienced clinicians are using Botox Unit (BU) to Dysport Unit (DU) ratio of 2.5 or 3:1. Stated another way, 20 BU is roughly the same in performance characteristics as 50–60 BU. We noted early after release of Dysport that 25 unit dosing showed rapid onset but early clinical effectiveness decay at or before 12 weeks in the glabella. In contrast, consistently greater than 12 weeks duration was seen at the 60 unit dosage for glabellar furrows. Apparently there was a reason Ipsen, Inc. (London, UK) produces Dysport in 300 unit vials (vs. 100 unit vials for Botox or 3:1).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








