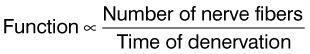33 Nerve transfers
Synopsis
 Nerve injuries are often devastating, with associated pain and impaired function.
Nerve injuries are often devastating, with associated pain and impaired function.
 Motor nerve injuries must be managed expeditiously, because regenerating axons must reach target muscle prior to degeneration and fibrosis – “time is muscle”.
Motor nerve injuries must be managed expeditiously, because regenerating axons must reach target muscle prior to degeneration and fibrosis – “time is muscle”.
 Nerve transfers offer an advantageous method of reconstruction by delivering regenerating nerve fibers to the target end organ more quickly, thus converting a proximal injury to a more distal injury.
Nerve transfers offer an advantageous method of reconstruction by delivering regenerating nerve fibers to the target end organ more quickly, thus converting a proximal injury to a more distal injury.
 Nerve transfers allow for dissection outside the original zone of injury, providing a safer and more technically straightforward procedure.
Nerve transfers allow for dissection outside the original zone of injury, providing a safer and more technically straightforward procedure.
 Unlike tendon transfers, the muscle–tendon biomechanical structure is preserved, thus excursion, origin, insertion, and length–tension relationships are undisturbed.
Unlike tendon transfers, the muscle–tendon biomechanical structure is preserved, thus excursion, origin, insertion, and length–tension relationships are undisturbed.
 Nerve transfers require time for the nerve to regenerate and extensive physical therapy for retraining.
Nerve transfers require time for the nerve to regenerate and extensive physical therapy for retraining.
 Intraneural dissection is technically demanding, and nerve transfers require intimate knowledge of nerve topography.
Intraneural dissection is technically demanding, and nerve transfers require intimate knowledge of nerve topography.
Key points
• Nerve transfers are a relatively recent development in peripheral nerve surgery.
• Nerve transfers can be performed to restore sensory or motor deficits.
• Nerve transfers essentially convert a proximal injury to a distal injury, providing a source of regenerating axons in close proximity to the end target.
• Nerve transfers are associated with minimal to no downgrade in donor function.
• Nerve transfers preserve the original biomechanical muscle–tendon relationship.
• Nerve transfers may provide superior results to tendon transfer, with the ability to restore individual and separate muscle function.
• Nerve transfers are replacing other techniques as the gold standard for brachial plexus and other peripheral nerve injury in the authors’ practice.
Introduction
Peripheral nerve reconstruction is an evolving field, and nerve transfers are an exciting addition to the surgical options. As our understanding of internal nerve topography improves, we are able to recognize redundant or nonessential potential donor nerves and fascicles. Recent contributions in basic science are broadening options for nerve transfer with new and improved techniques. This chapter will outline the history and principles of nerve transfers as they pertain to upper extremity nerve reconstruction. The recent literature as it relates to current techniques, debates, and advances in nerve transfers will be reviewed. Current commonly performed nerve transfers are detailed with focus on both motor and sensory nerve transfers, their indications, rehabilitation and postoperative protocols, and a basic overview of selected surgical techniques (see key points, above).
Historical perspective
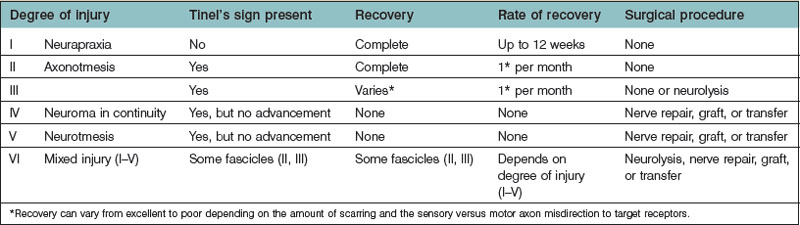
The classification of nerve injury is perhaps the single most important factor when determining management (Table 33.1). The six degrees of nerve injury have been well described.1–3 First- and second-degree injuries recover spontaneously, as do third-degree, albeit recovery is variable and less than normal, depending on scar tissue.4 Fourth- and fifth-degree injuries are significant and recovery is not expected. These injuries should be managed with prompt surgical intervention to prevent degeneration and fibrosis of the motor end plates.5 Sixth-degree injuries encompass a variety of nerve injuries within a single nerve, and the need to operate is determined on an individual basis.3,5
Table 33.1 Classification of nerve injury highlighting the six degrees of nerve injury and the anticipated rate and likelihood of recovery
Once surgical intervention has been deemed necessary, there are a number of surgical options. Traditionally nerve repair or grafting was used with tendon transfers to augment poor recovery or delayed presentation. The difficulty with proximal nerve injuries is that the distance from the injury to the motor end plate is significant, and recovery is prolonged. In addition, delayed intervention may result in denervation and fibrosis prior to muscle reinnervation.5 Axonal regeneration has been shown to occur at the rate of approximately 1 inch (2.5 cm) per month and reinnervation of denervated muscle is not possible after 12–18 months; therefore nerve transfers offer a valuable means of providing regenerating axons in closer proximity to the motor end plate.6 Essentially, a nerve transfer converts a proximal nerve injury to a distal one, enabling faster recovery and quicker return to function.
Nerve transfers were described very early; however they were traditionally reserved for otherwise unsalvageable root avulsions and proximal panplexus injuries.7 Approximately 20 years ago, nerve transfers were revisited and applied to a significantly broader patient population, with applications in partial plexus injuries to recreate specific essential functions in the upper extremity.3,8,9 As our understanding of internal nerve topography has broadened, techniques and equipment for performing microsurgery have improved, and our diagnostic ability for brachial plexus and peripheral nerve injury has progressed, the field of nerve transfers has exploded. Nerve transfers have become the standard of care for treatment of peripheral nerve injuries in the authors’ practice, and have largely replaced other modalities.
Basic science
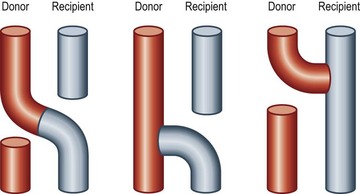
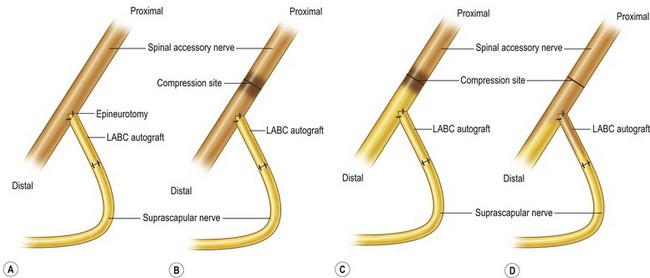
There have been dramatic increases in our knowledge of nerve injury, healing, and regeneration. The majority will be touched on in Volume 1, Chapter 22 and Chapter 32 of this volume. The most relevant recent basic science advances pertaining to nerve transfers relate to end-to-side transfers (Fig. 33.1). End-to-side neurorrhaphy involves the coaptation of the distal end of an injured recipient nerve into the lateral aspect of an intact nerve, which serves as a proximal source of axons to regenerate into the injured nerve. Reports of end-to-side transfers occurred as early as the late 1800s and then made a resurgence in the 1990s.10 There has, however, been controversy regarding the success of these transfers, and sensory recovery has typically been more impressive than motor recovery.11–15 The sprouting of axons in an end-to-side coaptation, thus eliminating the need for a proximal nerve stump, makes nerve transfers an option for proximal nerve injuries and has been well demonstrated in the literature.16–19 While sensory nerves are able to sprout spontaneously (collateral sprouting), donor nerve axonotmetic injury is required for motor neuronal regeneration (regenerative sprouting) across the end-to-side repair (Fig. 33.2).15,17,17,20,21 An epineurotomy in the donor nerve is required, and partial axotomy will result in more significant regenerative sprouting into the recipient nerve, albeit at the potential expense of a measurable downgrade in donor nerve function.17
Reverse end-to-side nerve transfers involve the complete transection of the donor nerve, which is then coapted into the side of the intact recipient nerve. This maximizes the potential number of available motor axons from the donor nerve. This procedure does not interrupt any recovery in the injured recipient nerve because the nerve remains in continuity; however the additional axons recruited from the donor nerve improve distal target reinnervation, a concept known as “supercharging.”22–24 In a proximal nerve injury, with a long distance required for reinnervation, this technique can protect target muscles from denervation atrophy and fibrosis.22
Diagnosis and patient presentation
History
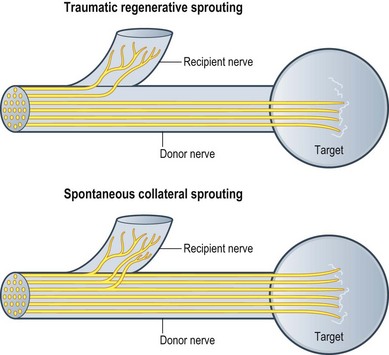
On history, details of the mechanism and timing of injury are crucial. The mechanism of injury will determine timing of intervention, with prompt exploration of penetrating sharp trauma, and more expectant management of closed injuries and gunshot wounds. Specific patient symptoms such as loss of function, both sensory and motor, and pain should be precisely elicited, as this will help focus the subsequent physical examination. The pain diagram and questionnaire are particularly useful for eliciting this information (Fig. 33.3). Other factors, such as patient age, handedness, occupation, and comorbidities, will factor into management decisions. When seeing a patient in a delayed fashion, it is also useful to ascertain any interval recovery of function.
Physical examination
Sensation should be examined by both dermatome and peripheral nerve distribution, and can be helpful in distinguishing these injuries. The authors advocate the use of both two-point discrimination and the ten-test to evaluate sensory loss in the hand.25 A Tinel’s sign, the tingling sensation elicited with percussion over a regenerating nerve, will help to localize the level of nerve injury, and may also be followed on serial clinical examination to check for signs of advancement, indicating spontaneous recovery. An advancing Tinel’s sign distal to the site of injury quite often precedes clinical motor recovery.
The scratch collapse test is useful primarily for patients with nerve compression pathology, but also has a role in evaluating the patient for potential nerve transfer procedures, as it can provide additional confirmation of the level of nerve injury (video 1). The test is performed by having the patient sit facing the examiner with the shoulders adducted, elbows held in 90° of flexion, neutral prosupination, and wrist and fingers extended. The examiner will then lightly scratch the area of the presumed nerve injury and exert force to the patient’s arms in the direction of internal shoulder rotation as the patient resists. Nerve injury at the test site is indicated by the inward collapse of the arm on the side ipsilateral to the injury.26,27
Imaging
Imaging is primarily useful for confirming level of injury in more complex closed-mechanism nerve injury patients. For example, imaging such as computed tomography myelogram and magnetic resonance imaging provides additional confirmation of a nerve root avulsion pattern. Additional X-rays, such as shoulder films that demonstrate scapular notch-level trauma, may provide information of localized trauma that would make proximal transfer to the suprascapular nerve ineffective due to the extensive zone of injury. Other plain films such as chest X-ray might be used to evaluate pathology such as rib fractures or diaphragm dysfunction that might make the use of intercostal and phrenic nerves as donor nerves less palatable, although the former is debatable.28
Electrodiagnostic testing
Electrodiagnostic testing, performed by an experienced person, can be a useful adjunct to physical examination for serial assessment of reinnervation in closed nerve injuries. Electromyography records the electrical activity of muscle fibers, tested both at rest and activity, by the insertion of needles into the muscle. Denervated muscles will demonstrated fibrillations and positive sharp waves on needle insertion; however the findings are not reliable until approximately 4 weeks after injury.29 Initial electrodiagnostic testing should be deferred until a minimum of 6–8 weeks postinjury to assess for signs of both axonal injury and root-level avulsion. Serial testing will reveal signs of reinnervation with nascent potential and motor unit potentials in the affected muscle groups, often prior to clinical evidence of recovery. Conduction velocity is used to measure the integrity of a peripheral nerve (sensory nerve action potentials (SNAP) or compound motor action potentials (CMAP)).29 A severed nerve will lose the ability to conduct a signal as wallerian degeneration occurs; however, if measured too early, the distal aspect of the nerve will still conduct, leading to an inaccurate assessment. This is another reason for delaying initial electrodiagnostic studies. Preganglionic injuries will be associated with a loss of sensation but an intact SNAP and an absent CMAP.29 Thus the level of injury can be confirmed with electrodiagnostic studies.
Overall, the complete picture, including history, physical examination, and adjunct testing, should facilitate surgical decision-making. Appropriate treatment for open injuries with nerve dysfunction begs for timely management with surgery on an urgent or semiurgent basis. For these injuries, direct exploration and repair have historically been the treatment of choice, but in very proximal injuries, distal nerve transfer can allow for more timely and successful reinnervation. In closed nerve injuries or in gunshot wounds, appropriate management demands a more measured approach and surgery should be delayed to allow for spontaneous recovery, which is often superior to that seen in patients treated with hasty surgery. These patients in particular may benefit from “supercharging” with reverse end-to-side nerve transfer procedures, which can more quickly deliver regenerating axons to the appropriate end organ, while still allowing slower spontaneous recovery.22
Patient selection
Nerve transfers have become increasingly popular for a number of reasons, the most compelling of which is the “time is muscle” issue.24 The biggest challenge facing peripheral nerve surgeons is that with increasing time since injury, the ability to achieve good motor function becomes increasingly limited. In fact, for any nerve injury where there is complete discontinuity with the motor end organ, no reinnervation procedure will be able to restore muscle once denervation and fibrosis have occurred, a process which occurs as early as 1 year.24 Nerve transfers, by bringing the regenerating motor fibers closer to the target end organ more rapidly, essentially convert a more proximal-level injury to a more distal-level injury, thus increasing the chance of achieving meaningful muscle function.
Another advantage of nerve transfers is that they enable surgical reconstruction outside the zone of the original injury, avoiding complex dissections and limiting injury to critical neurovascular structures. In patients with brachial plexus trauma, the proximity of vital structures (great vessels, thoracic duct, lung) makes the occurrence of potentially life-threatening complications a real possibility. In other cases, such as concomitant vascular injury, previous blast injury, or soft-tissue deficits requiring flap reconstruction, the ability to avoid doing a complex nerve exploration in the setting of dense fibrotic scar is particularly advantageous.24
Nerve transfers allow for a very targeted intervention in cases of partial nerve injury such as in treatment of a sixth-degree or neuroma-in-continuity injury, where transfer can be done distal to the site of injury specifically to the nonfunctioning nerve branch. This allows for preservation of all intact function and restoration of only missing function. In addition, the ability to “supercharge” a recovering nerve is invaluable when it is unclear whether the nerve transfer or intrinsic recovery will be more beneficial.14,30
Unlike tendon transfers, nerve transfers require only minimal immobilization (7–10 days), which is especially valuable in patients presenting with significant baseline stiffness. Nerve transfers also preserve the biomechanical properties of the musculotendinous unit, such as end organ origin, insertion, excursion, and length–tension relationships. Finally, nerve transfers can restore unique function such as pronation, which is incredibly difficult to restore by traditional surgical techniques.31
Thus nerve transfers are indicated in a number of situations (Table 33.2), for example, in proximal brachial plexus injury where grafting is not possible or the required distance for reinnervation will not allow motor axons to reach the target motor before denervation and fibrosis have occurred. In situations of extreme scarring, or major upper extremity trauma, a nerve transfer is preferable to risking damage to critical structures within the scarred region. Nerve transfers are also indicated in segmental nerve loss, and in partial nerve transfers with functional loss. Patients presenting in a delayed manner with inadequate time to reinnervate the distal targets are good candidates for distal nerve transfers. Also, patients with sensory nerve deficits in critical regions should be considered for nerve transfer to restore sensation.
Table 33.2 Indications for nerve transfer
Examples of nerve transfer procedures for specific injury patterns
Hints and tips
• Avoid, or use short-term paralytics with anesthesia induction to allow for nerve stimulation
• Minimize or avoid tourniquet time to avoid interference with nerve stimulation
• Use plain epinephrine in proximal incisions to minimize blood loss without lidocaine paralysis
• Obtain wide surgical exposure to identify nerves and appropriate branches
• Choice of optimal nerve donor is based on quantity of motor axons, proximity to target muscles, synergy of muscle function, and donor expendability
• Conduct neurolysis with your “eyes” except at the site of actual transfer to avoid prolonged dissection and increased trauma to nerve branches
• Confirm no intraoperative stimulation in putative recipient before dividing donor
• Divide donor nerve distally and recipient nerve proximally
• Use 9-0 nylon and the operating microscope to perform tension-free epineurial repair
• Use bupivacaine block at end of case for postoperative pain control
Upper plexus injury
Reconstruction techniques
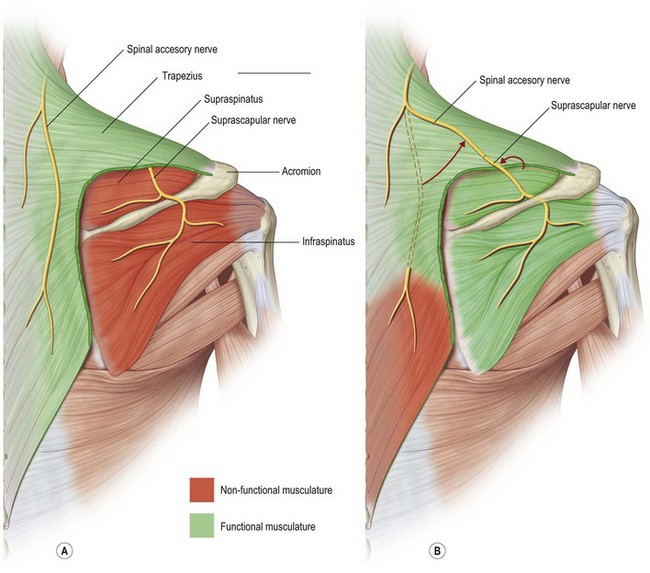
Use of spinal accessory nerve (cranial nerve XI) to suprascapular nerve transfer (motor)
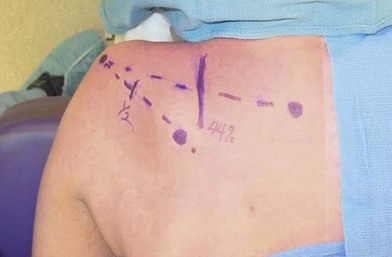
Restoration of shoulder stability and external rotation are facilitated by transferring the spinal accessory nerve (cranial nerve XI) to the suprascapular nerve. This transfer can be conducted by either an anterior or a posterior approach. In the posterior approach, the patient is positioned prone and surface landmarks are used to approximate the position of the nerves (Fig. 33.4). The spinal accessory nerve runs parallel to the border of the trapezius and is localized 44% of the way along a line connecting the acromion to the dorsal midline at the level of the superior border of the scapula (Fig. 33.5). The suprascapular nerve is located midway between the medial border of the scapula and the acromion as it runs through the suprascapular notch.32,33 These nerves are accessed through an incision located slightly obliquely just above the superior border of the spine of the scapula. Dissection is carried through the trapezius in a muscle-splitting fashion and an end-to-end coaptation, sparing the upper trapezius nerve branches, is performed.
For the anterior approach, an incision is designed 2 cm superior to and parallel to the clavicle extending laterally from the posterior border of the sternocleidomastoid (Fig. 33.6). The upper trunk is identified between the anterior and middle scalene muscles. The suprascapular nerve is a distinct branch of the upper trunk that sits on the superolateral aspect. The spinal accessory nerve is located in the posterior aspect of the incision on the deep surface of the trapezius muscle. Although an end-to-end transfer can be performed, the end-to-side approach with a partial neurectomy of the donor accessory nerve is preferred as this preserves some donor function. In the end-to-side transfer, a short interpositional graft from the recipient suprascapular nerve to the donor spinal accessory nerve is required to avoid tension. A proximal crush injury to the donor nerve encourages axonal sprouting such that regeneration into the recipient suprascapular nerve occurs without loss of significant donor nerve trapezius function.14
Use of triceps to axillary nerve transfer (motor component)
Additional reduction of glenohumeral subluxation and abduction of the shoulder are provided by transferring a branch of the triceps, usually from the medial head, to the axillary nerve (Fig. 33.7).33,34 Better results in upper plexus injury patients are achieved by reinnervating both the suprascapular and axillary nerves.35 This transfer is performed with the patient positioned prone through a longitudinal incision on the posterior surface of the arm that extends in a curvilinear fashion above the posterior axillary fold (Fig. 33.8)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree