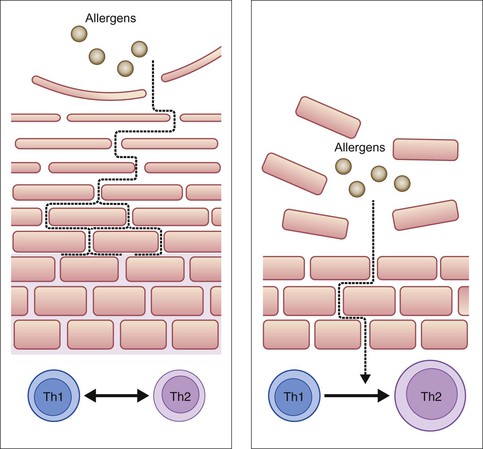
Simon G. Danby, Carol Bedwell, Michael J. Cork The skin of the neonate has a defective skin barrier relative to an older child. The neonate is extremely vulnerable to damage by environmental agents such as harsh detergents, some topical oils and other irritant chemicals. These agents can interact with genetic tendencies such as FLG gene variants1 and lead to the development of compromised barrier function, and may contribute to the development of atopic dermatitis (AD).2 For some infants, this is the first step along the atopic march; the development of one or more of food allergy, allergic asthma, and allergic rhinitis.3 Evolving perspectives on barrier dysfunction in neonates has led to the idea that there may be a window of opportunity in the first few months of an infant’s life to change the environmental agents that their skin is exposed to, in order to maximize skin health, prevent the breakdown of the skin barrier, reduce the development of AD and potentially minimize the atopic march.4 These environmental changes could involve the use of optimally formulated wash products, emollients and the removal of all other irritant substances that could damage the skin barrier. Early clinical trials work has been performed, and future research may guide optimal infant skin care from birth.5 As discussed in Chapters 1, 2 and 4, there are structural and developmental differences in the skin of adults, full-term infants and premature neonates. The epidermis develops throughout the second trimester of pregnancy and reaches structural maturity in the third trimester, somewhere between 30 and 37 weeks’ gestation.6 At this time, a well-defined stratum corneum (SC), or ‘horny layer’, is observed. The intact SC forms the primary barrier to the penetration of irritants, allergens and invading bacteria through the skin, henceforth referred to as the skin barrier.4 At about 26 weeks’ gestation, the epidermis is thin, consisting of only a few cell layers with a poorly formed SC.7 Skin barrier function is positively correlated with gestational age during the period of SC maturation.8 For instance transepidermal water loss (TEWL), an indicator of permeability barrier function,9 is 10–15 times higher at 25 weeks’ gestation compared with a term infant. Infants less than 24 weeks’ gestation have a minimally protective SC at birth, where TEWL is similar to the free evaporation of water.7,8 Under these conditions, evaporative heat loss is thought to exceed resting heat production. In addition, their ratio of body surface area to weight is up to five times that of an adult.10 Premature infants also have an attenuated layer of subcutaneous fat and nonfunctional eccrine glands at birth, which together lead to compromised thermoregulatory capabilities.11 After abrupt exposure to the xeric, postnatal world, premature skin matures rapidly over 2–8 weeks, taking significantly longer for the most premature and lowest-birthweight neonates.12 Until the epidermis reaches maturity, premature infants are much more susceptible to fluid/electrolyte disturbances, percutaneous absorption of topically applied agents, and percutaneous infection, as a result of inadequate skin barrier function.7 Moreover, while the SC is generally considered ‘visually mature’ in the full-term neonate, recent evidence suggests that the skin barrier does not achieve ‘optimum’ performance (or functional maturity) for a number of months following birth.13 This period of adaption to the extrauterine environment is characterized by a number of changes in the biophysical properties of the skin that differentiate it from adult skin. Structurally, the thickness of the SC in infants aged up to 24 months is on average 30% thinner when compared with adults.14 In addition, the thickness of the supra-papillary epidermis is 20% thinner. Similar, lower, and higher TEWL readings have all been reported at different body sites in groups of infants at varying ages compared with adults. Differences in methodology, equipment, test site, and the study populations all contribute to the disparity between TEWL measurements.2,8,13 Furthermore TEWL readings in infants are more varied compared with adults, suggesting that infant skin is in a state of flux.15 Nikolovski and colleagues reported that, in addition to elevated TEWL on the forearms, water absorption and desorption rates were higher, and that there is an altered distribution of water throughout the SC.15 SC hydration (SCH) is reduced in term infants compared with adults, but increases with increasing postnatal age, resulting in higher, more varied, levels in infants (3–12 months old) compared with adults.8,13 The water-handling properties of the infant SC appear to be very different to those of an adult. One of the causes of this may be the reduced amount of Natural Moisturizing Factor (NMF) found within the SC of infants compared with adults.15 NMF is a collection of hygroscopic compounds involved in moisture retention, the levels of which correlates with TEWL and SCH/clinical dryness.16–19 Components of NMF, including urocanic acid and lactic acid, also play a role in maintaining the acidic pH of the skin surface.16,17,20 Skin surface pH is neutral at birth in humans and only gradually develops an acid pH over a period of 28 days, and in some cases, may not reach normal/adult pH levels until 2 years of age.8 SC pH is a key factor governing skin barrier homeostasis, including regulation of desquamation and lipid synthesis.21,22 Accordingly, the size of the uppermost corneocytes is 20% smaller in infants (3–24 months) compared with adults, indicating an increased rate of desquamation.14 The composition and type of lipids in the SC appears to be dynamic during infancy, not least because of the reduced production of sebum before puberty.23,24 Changes in the make-up of the lipid lamellae affect its structure and subsequently, its ability to act as a permeability barrier.23 Furthermore, certain SC lipids possess important antibacterial properties, the SC levels of which are associated with the skin’s susceptibility to infections.25 Coupled with an elevated skin surface pH, which creates a more favorable environment for the growth of pathogenic bacteria, infant skin exhibits a suppressed antimicrobial barrier, which fits with the increased occurrence of skin infections in infants compared with adults.8,25 Taken together, these changes in the biophysical and biological properties of infant skin indicate that the skin barrier undergoes a period of optimization and adjustment over a period of about 12 months, depending on the body site.2,13 During this time, the skin is more susceptible to irritant and allergen penetration and bacterial colonization (Fig. 5.1). An inverse relationship has been reported between postnatal age and the percutaneous absorption of isotope-labeled benzoic acid.26 Furthermore, infant skin, generally considered to be ‘sensitive’, is prone to the development of dermatological conditions including allergic/irritant contact dermatitis and acne infantum.12,27 The temporary retardation of skin permeability and antimicrobial barrier function also coincides with the most at-risk period for developing AD, which affects one in five children.28,29 Of AD cases, 45% arise during the first 6 months of life, and 60% by the first 12 months.30 Furthermore, Illi and colleagues reported that 43.2% of children with early onset AD were in complete remission by 3 years of age.31 This may reflect the progressive optimization of the skin barrier over the first months to years of life (Fig. 5.2). A number of genetic and environmental factors are known to interact producing negative consequences for the structure and function of the skin barrier,32 especially during the period of postnatal skin development. FLG gene mutations resulting in loss of functional filaggrin and its downstream products are the most significant genetic risk factors for the development of AD identified so far, and account for between 15% and 50% of cases, dependent on severity.1,33 Carriers of FLG loss-of-function mutations with AD exhibit a more pronounced skin barrier defect, characterized by elevated TEWL, increased skin surface pH and decreased SCH, compared with AD patients without these mutations.17,18 Having an FLG loss-of-function mutation likely affects the development of the skin barrier from birth, but alone may not lead to AD; additional genetic and environmental factors are implicated (Fig. 5.3).33 Washing the skin with soap and harsh detergents is known to exacerbate AD and other dry skin conditions, especially where the wash water is hard.34,35 A single wash with soap or the anionic surfactant sodium lauryl sulphate (SLS) elevates TEWL and skin surface pH and decreases SCH. Washing with hard water leads to increased soap use and soap deposition on the skin, leading to increased dryness (decreased SCH) and irritation.36 Furthermore, hard water contains high levels of free calcium, which has been shown in vitro to inhibit skin barrier repair.37 Combining soap or harsh detergents with hard water is thought to result in exaggerated skin barrier damage and prolonged recovery, which may facilitate the development of conditions involving a skin barrier defect. Indeed, living in a hard water area is significantly associated with increased risk of developing AD.38,39 A clinical trial in 2008 reported no effect on the severity of AD associated with the installation of a water softener.40 The study did not address, however, the role of wash product interaction with the wash water, the effect of the intervention on development of AD or the nature of the intervention itself (the water softening systems used do not remove all calcium ions, unlike ultrapure water softeners, and do not alter the alkalinity of the wash water). Further research is required to elucidate the role of water hardness. Current guidelines for the treatment of AD recommend the avoidance of soap and harsh detergents, such as SLS along with other negative environmental factors.41,42 In infants, saliva, nasal secretions and some foods are important environmental factors that interact with genetic variants to exacerbate the skin barrier defect.43 Saliva contains proteases and lipases and has a pH of 7.42 (±0.4) with a substantial buffering capacity.44 This provides an optimal pH for protease activity and therefore facilitates skin barrier breakdown. Similarly, breast milk has a pH of 7.29 (±0.19),45 and nasal secretions have a pH of 6.91 (±0.06),46 optimal for protease activity. The combination of saliva, breast milk and nasal secretions caught under a pacifier in a baby with AD leads to a localized ‘drooling dermatitis’ around the mouth and onto the cheeks.43 A combination of adverse environmental factors also contributes to diaper (napkin) dermatitis; urine and feces in conjunction with diaper occlusion, yields elevated skin surface pH and are the sources of additional degradatory proteases and lipases.47 The interaction of these negative factors at the diaper area has been shown to affect development of the skin barrier at this site. Drooling dermatitis may play a role in the development of food allergy. The route of sensitization to some food allergens, such as peanuts, is thought to be through the skin rather than by ingestion.48 This supports the observation that the development of AD is often linked to the development of other atopic conditions, including food allergy and asthma.3 The increased risk of further progression along the atopic march highlights the importance of more effective treatment of dermatitis in infants. In the case of drooling dermatitis, avoiding pacifiers and the use of barrier emollients to reduce epidermal damage by saliva (and some foods) should be combined with effective cleansing of the face using products that do not damage the skin barrier. The continued development (optimization) of the skin barrier and the interplay of negative environmental factors affecting infant skin, raise the importance of how our skin is cared for during the first 2 years of life. While we cannot change the genetics of a neonate or infant, we can modify environmental exposures to help prevent further damage to the skin barrier. For both children and adults, bathing is a hygienic necessity. The goal of hygiene is the preservation of skin health. However, there is a balance between cleansing the skin and preservation of its homeostatic properties such as skin barrier integrity.49 Cleansing is essential to remove pathogenic bacteria such as those from feces. Feces, saliva and other body secretions contain enzymes such as lipases and proteases that breakdown the skin barrier. However, frequent bathing of infants is more of a cultural and aesthetic practice that allows for tactile interaction with the caregiver. Benefits must be carefully considered along with the detrimental effects that can occur with bathing, especially in premature infants.49,50 A balance needs to be reached between the frequency of bathing to achieve optimal cleansing and maintaining an intact skin barrier. For neonates, bathing can lead to hypothermia, increased oxygen consumption, respiratory distress, and destabilized vital signs.51 Therefore, the first bath should be delayed until after vital signs and temperature have remained stable for at least 2–4 h.52,53 Immersion bathing, when feasible, may be beneficial from a developmental perspective. It is more soothing, and can promote enhanced sleep,54,55 and in studies, was not associated with significant differences in oxygen saturation, respiratory rate, or heart rate in premature infants of less than 32 weeks’ gestation.56 The water level should be high enough to cover the infant’s entire body to aid temperature control and decrease evaporative heat loss. The optimal temperature of bath water is 100.4°F, which should always be accurately measured. Second-degree burns have been reported after immersion into overly hot bath water tested only by touch.57 Sponge bathing with a moistened cotton ball or cloth is an acceptable alternative. Immediately after bathing, the infant’s skin should be gently towel dried and a head covering applied.52 Vernix caseosa, composed of sebaceous gland secretions, desquamated skin cells, and shed lanugo hairs,58 is negligible in preterm infants. Vernix need not be removed,49 as this layer may aid in thermoregulation, hydration, bacterial protection, and wound healing.58–61 The first bath should be given respecting universal precautions to limit contact with transmissible pathogens. The optimal frequency of bathing is open to debate.62–64 Research suggests that bathing twice a week will not affect TEWL or skin surface pH, assuming that mild products are used,65 and as such, Blume-Peytavi and colleagues recommend that bathing take place no more frequently than every other day.13 The use of water alone or water with added skin cleaning products for bathing has led to considerable debate.66 Soaps and cleansers containing harsh detergents, such as SLS increase skin surface pH and reduce SCH.67 Conversely, washing with tap water alone leads to the extraction of water-soluble metabolites from the skin, including NMF, and causes mild irritation and increased skin dryness. Furthermore, water alone is ineffective at removing fat-soluble substances from the skin.68 The surfactants in cleansers are required to remove these potential fat-soluble irritants. A balance is therefore required between effective cleansing and the need for the mildest surfactants.69 SLS is a very effective cleanser, however it is very irritating to the skin, partly associated with its alkaline property. Wash products designed for use on babies’ skin contain very mild surfactant complexes and are buffered to skin pH in order to minimize the negative effects on the skin barrier associated with alkaline wash products.69 The use of cleansing products compared with water alone for bathing newborns has been subject to a systematic review,70 which focused on two trials. One study71 comprised of four trial arms, including washing twice weekly with a wash gel product; bathing twice weekly with water, then applying a cream; bathing with wash gel and applying cream after bathing; and bathing with water only. The second study72 compared two different liquid cleansers with water for bathing infants. The results of these studies indicated no harm to skin barrier function with the tested cleaning agents, although the number of babies in each treatment arm was small.70 A large UK-based trial of a mild pH 5.5 liquid SLS-free cleanser versus water alone in bathing neonates for the first 28 days showed essentially no differences in TEWL, hydration, skin surface pH and clinical observations.73 Skin hydration was raised in the cleanser arm at 14 days postnatal (ITT: mean wash score 49.7 (SD 9.2) vs water 46.7 (SD 8.4), p = 0.02, 95% CI −5.46 to −0.43), but was equal to those in the water group at 28 days postnatal. There have been no studies to support the routine use of antiseptics. The once-conventional use of antimicrobial cleansers for routine infant bathing diminished following recognition of the toxicity risks. Hexachlorophene was widely used prior to 1975, and was subsequently associated with serious adverse reactions in infants, including fatal neurotoxicity. There has been only one subsequent report of an infectious complication possibly linked to a change in bathing with nonantiseptic cleanser.74 The other significant problem with any form of antiseptic is that it can interfere with the acquisition and evolution of normal flora, which may influence colonization with pathogenic bacteria and/or fungi.75,76 Care of the infant scalp and hair follows the same principles as skin care. The same or similar gentle liquid cleansers may be used.77 ‘Baby’ shampoos are distinguished by a low ocular irritation index and a pH and saline concentration similar to those of tears.49 This is achieved by using very mild surfactant complexes and buffers in a similar approach to body wash products.78 Allergic contact dermatitis from cleansing products, including shampoos, is rare,79 as they have very brief contact with the skin before being rinsed off. Minimal nail care is needed in the neonatal period. Fingernails should be trimmed to limit self-inflicted excoriation, avoiding close trimming that can cause bleeding and lead to infection.80 Emollients in their simplest form produce a layer of oil over the surface of the skin that traps water underneath it. This water passes back into the dehydrated corneocytes and as a result, they swell up closing gaps between the corneocytes. This is therefore purely an artificial and temporary repair of the defective skin barrier,81 as such effective use requires frequent application on about 150–200 g per week in young children.42 An ointment containing just oil is the simplest form of emollient; examples include liquid paraffin, white soft paraffin, yellow soft paraffin and mixtures such as 50/50 white soft paraffin and liquid paraffin. These mineral oils are inert, are highly purified and as a result, are extremely unlikely to cause cutaneous reactions.82,83 This is why mineral oils are the basis of the majority of emollient formulations and used as the base of many medicinal and wash products. Liquid paraffin is useful as an emollient and massage oil and is not very occlusive. In contrast, white soft paraffin is very occlusive and can lead to folliculitis eruptions, especially in hot weather. As soon as water is added into a formulation, whether it is an ointment or cream, then emulsifiers are needed to form a stable formulation of the oil and water. Emulsifiers are surfactants (detergents) and as a result, can damage the skin barrier.69 The very harsh anionic surfactant SLS for example, solubilizes SC lipids, denatures keratin and elevates skin surface pH resulting in disturbed skin barrier homeostasis. It is therefore reasonable to use emollient cream and ointment formulations that contain the mildest emulsifiers, which will cause the least damage to the skin barrier.84 Some emollients, creams, and ointments contain SLS, despite its use as a standard skin irritant in patch-testing. Examples include aqueous cream BP, emulsifying ointment and many proprietary emollients. The use of aqueous cream BP as a topical emollient was found to damage the skin barrier; an effect associated with the presence of SLS in its formulation.84–86 Furthermore, aqueous cream BP was associated with irritant cutaneous reactions and exacerbation of the condition in the majority of infants and children with AD in a pediatric dermatology clinic.87 The effect of a simple petrolatum cream can be enhanced by adding humectants such as glycerol, urea, lactic acid and sodium pyrrolidone carboxylic acid, some of which are naturally found in the skin as part of NMF.88–90 Humectants attract and hold water in the SC,91 and therefore compensate for the reduced levels of NMF found in infant skin. The ability of an emollient to repair a defective skin barrier may also be enhanced by the addition of ingredients that contribute to enhancement of the lipid lamellae, including ceramides and particular fatty acids such as linoleic acid.81 The use of emollients is, in general, associated with improvement in skin dryness, pruritus and skin barrier function.41 However, there is a very limited evidence base for the efficacy of emollients.42,92 Randomized controlled trials published in recent years in established AD suggest that the use of certain ‘complex’ emollients can be steroid sparing, reduce the severity of AD and delay relapse of the condition.90,93 Importantly, emollients are not all the same, and depending on their formulation can have very different effects on the skin (Table 5.1). While aqueous cream, with an old formulation comprising a harsh surfactant and no humectant, is minimally hydrating and damages the skin barrier, newer more sophisticated formulations containing humectants considerably improve hydration while being mild. It is the positive effects of certain emollients that has raised the possibility of emollient therapy from birth as a promising intervention for the prevention of AD in high-risk cases.94 TABLE 5.1 Key differences between emollients Infant massage, a tradition in many cultures, has recently been repopularized. Infant massage has many reported and theoretical benefits, including increased interaction and bonding opportunities between infants and parents, improved parental understanding of the infant’s behavior, and reduced parental stress.95,96 Variables studied in hospitalized premature infants include decreased motor variability and distress, and enhanced weight gain and development, leading to reduced length of stay.97 However, an evidence-based review in 2004 did not recommend this practice,98 calling into question the methodological quality of the studies. Interestingly, several studies have suggested that weight gain associated with infant massage may be due primarily to percutaneous absorption of essential fatty acids from the massage oil. Animal studies first documented a reversal of fatty acid deficiency in rats treated with cutaneous application of essential fatty acid (EFA)-rich safflower oil.99 This was not reproduced in neonates treated with sunflower seed oil,100 but subsequent studies in premature and full-term infants have shown positive effects of oil massage. Weight and length gain velocity were statistically increased in newborns receiving oil massage four times a day for the first 31 days of life.101 Infants massaged with safflower oil had significant rises in serum essential fatty acids, including linolenic acid and arachidonic acid, whereas infants massaged with coconut oil had increases in saturated fats.102 Certain natural oils have also been reported to soften the skin and provide moisturization,103 possess antimicrobial activity,104–106 display anti-inflammatory properties107,108 and reduce skin irritation.109 Randomized controlled trials on the clinical effect of topically applied sunflower seed oil and almond oil have reported significant benefits, such as reduced nosocomial infections and quicker recovery of dermatological conditions.104,105,110,111 These benefits can be, at least in part, linked to improved skin barrier function.110,112 Notably however sunflower seed oil was found to improve skin barrier integrity, whereas olive oil had the opposite effect, being associated with skin barrier damage and mild irritation.112–114 When a panel of 14 natural oils were compared for their ability to prevent experimentally induced irritant contact dermatitis in humans, positive effects (based on clinical scoring of irritation, TEWL and chromametry) were associated with oils containing low oleic acid and high linoleic acid.115 Olive oil contains 55–83% oleic acid, a skin penetration enhancer that at 5% significantly increases TEWL when applied to the skin.116 In contrast to olive oil, the predominant fatty acid in sunflower seed oil is linoleic acid. Linoleic acid has been shown to exert positive effects on the skin barrier,112 attributed to its potent activation of peroxisome proliferator-activated receptor α (PPARα), involved in regulating keratinocyte proliferation, inflammation and skin barrier homeostasis. The choice of massage oil therefore has significant consequences for the condition of the skin and the risk of bacterial sepsis. The type of oil used varies according to country. This has led to a number of investigations of the impact of different types of oils (e.g., mustard, almond, sesame, herbal, mineral, coconut, vegetable and sunflower seed) on newborn skin.104,111,117–121 In the UK, a recent survey of oil use found that 52% of maternity/neonatal units recommend the use of oil for infant skincare.122 Olive oil was most frequently recommended (82%) followed by sunflower seed oil (21%). Further qualitative work found that midwives and health visitors regularly recommend the use of natural oil for babies’ dry skin.66 Their preference is influenced by their belief in the safety of natural oils and the desire to offer a cheap solution to the problem of dry skin. This opinion is in the presence of evidence indicating that olive oil causes significant damage to the skin barrier. In view of the positive effects of mineral oil on the skin barrier82 an RCT comparing its use with other oils is needed. In light of the positive and negative effects of topical massage oils, their complex and variable composition, and the potential for sensitization,123 further research is required to ensure that the oils used for massage are safe and effective. The first disposable diapers marketed in 1963, had an absorbent core of cellulose fluff. In the mid-1980s, a superabsorbent core material containing a cross-linked sodium polyacrylate was developed. This material, contained in all superabsorbent disposable diapers, transforms and holds fluid within a gel and has the capacity to absorb many times its own weight. As a result of this, pseudoanuria has been reported in an infant, because of the inability to feel moisture on a superabsorbent diaper.124 Urine output, monitored by weighing diapers, can also be erroneous if diapers are allowed to remain open under a radiant warmer.125 Irritant diaper dermatitis is rare in the immediate neonatal period, but increases in incidence over the first month,126 with overall prevalence between 4% and 15%.127 There is a complex interplay in the occlusive diaper environment, with many components contributing to the pathogenesis of diaper dermatitis. Excessive hydration leads to maceration and increased skin permeability. Now prone to injury, the addition of alkaline urine changes the normal protective acidic pH of the skin128 and permits the growth of microorganisms, including Candida albicans, Staphylococcus aureus (S. aureus), and Streptococcus.129 This pH also activates fecal lipases, endogenous and exogenous proteases, and bile salts, which can lead to further injury.21,130 Superabsorbent diapers are clearly superior to cloth diapers in preventing irritant diaper dermatitis.131 Disposable diapers effectively reduce maceration, help create a favorable pH, decrease exposure to urine and feces, and better contain enteric pathogens.132,133 Other contactants in the diaper area may not have such a favorable profile and can worsen the factors that lead to diaper dermatitis. Topically applied medications in the diaper area are also more readily absorbed and can lead to irritation, sensitization, and percutaneous toxicity. The use of medicated baby wipes has increased over recent years for diaper area cleansing of term newborns.134 However, concerns have been raised related to the suitability of these products and their potential links to dermatitis.135 A wide range of baby wipe products are available, with varying formulations.136 Older formulations, in particular, include harsh surfactants, alcohol and fragrance, which have been associated with contact dermatitis in adults.135,137,138 More recent product development has led to wipes free from alcohol, fragrance, with appropriate surfactants and preservatives, and pH adjusted to 5.5. These more recent formulations have been subject to clinical trials with newborn infants. Two small scale studies139,140 indicated that wipes are suitable for use with term infants in the newborn period, but were methodologically limited by sample size. This has been confirmed by a recent assessor-blinded randomized controlled trial by Lavender and coworkers,136 focusing on healthy term neonates (n = 280) from birth to 28 days postnatal. This study found one wipe formulation, with low pH 5.5 and alcohol and fragrance free, had an equivalent effect to water on skin hydration, TEWL or pH versus water. While dermatitis has always been a concern, there was no evidence of any increase in erythema or skin breakdown in any of the clinical measures used, although data from maternal observations of diaper area skin suggest a higher rate of low-grade diaper rash in babies cleansed with water and cotton wool (p = 0.025). The authors conclude that the wipe formulation used in the trial (pH 5.5, alcohol free, fragrance free) is equivalent to water and cotton wool for neonatal diaper area cleansing. The overall density of melanocytes in skin is greater in children than in adults, but melanin production is limited and melanocytes in children may be more susceptible to ultraviolet (UV)-induced damage.141 In addition, infants and children have not had the gradual UV exposure that stimulates facultative pigmentation. For these reasons, pediatric patients are more susceptible to the damaging effects of excessive exposure to sunlight. A sunscreen is a compound that absorbs, reflects, or scatters the harmful spectrum of UV light (290–400 nm). An increasingly wide variety of sunscreen products are available. Ingredients that reflect and scatter a large portion of the solar spectrum, including UVB, UVA, and visible light, are zinc oxide and titanium dioxide. The safety of topically applied sunscreens has not been established for infants under 6 months of age, but the theoretical risk of toxicity is low. Concerns in the past have focused on neonatal metabolism of p
Neonatal Skin Care and Toxicology
Introduction
Structure and function of neonatal skin
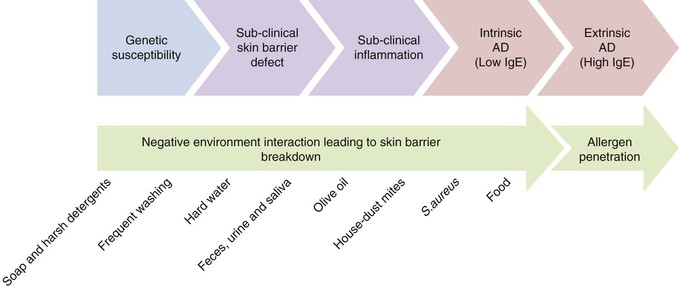
Gene–environment interaction
Skin care practices
Skin cleansing
Emollients
Properties of dry skin in infants
Different properties of emollients
Effects on the skin
Reduced levels of natural humectants (NMF)
Humectant (such as urea, glycerol and lactic acid)
Humectants bind to water in the SC and improve the hydrating effects of emollients
Abnormal lipid lamellae
Physiological lipids (including ceramides, cholesterol and free fatty acids)
Some physiological lipids help repair the defective lipid lamellae and improve skin barrier function
Increased susceptibility to irritants
Surfactant system (required to emulsify oil in water formulations)
Harsh surfactants like SLS can damage the skin barrier and irritate the skin, whereas some surfactant complexes are as mild as water
Elevated skin surface pH
Product pH and buffering capacity
Modification of skin surface pH with subsequent effects on skin barrier homeostasis. Products that increase skin surface pH are associated with increased proteolytic breakdown of the skin barrier.
Infant massage
Diapering and diaper care products
Ultraviolet protection and thermal injury
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Neonatal Skin Care and Toxicology
5
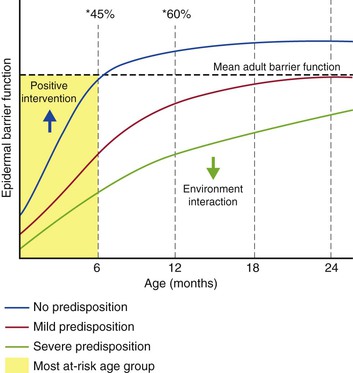
Figure 5.2 An illustrative representation of the proposed development of AD with age. Skin barrier function, assessed by TEWL, does not achieve adult status until after 1 year of age.15 The SC does not appear to reach maturity on average until after 24 months.14 The determination of mean adult barrier function (dotted line) is based on normal-appearing volunteers (no clinical signs of AD) who may or may not have an underlying predisposition to a defective skin barrier. A predisposition to a defective skin barrier, the severity of which is thought to be dependent on gene–gene (and gene–environment) interaction, is thought to delay skin barrier development (green and red lines), compared to having no predisposition (blue line). The function of the fully developed barrier is similarly dependent on the inherited predisposition and will determine the life-long susceptibility of an individual to environmental insults. *45% of AD cases will arise by the age of 6 months, and *60% by 12 months.30 The prevalence of AD in the first 2 years of life is 21.5%.31 Positive intervention that shifts skin barrier function towards adult levels is expected to decrease the risk of developing AD (blue arrow).