Fig. 6.1
(a–b) Coexistent nasal polyposis, frontal mucoceles, and inverted papilloma (IP). Note the fleshy looking appearance of the IP that is different from the edematous pale nasal polyps
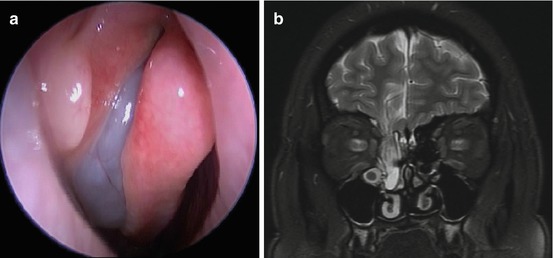
Fig. 6.2
(a) A meningoencephalocele can mimic the endoscopic appearance of a middle meatal polyp. (b) MRI revealing the presence of the meningoencephalocele
Imaging
Computed tomography (CT) of the paranasal sinuses remains the primary imaging modality for evaluating patients with nasal polyposis. Signal heterogeneity within the sinuses, best observed on soft tissue windows, may indicate underlying fungal sinusitis, secondary to the presence of metals such as iron or manganese, or calcium precipitates within the mucin. CT is excellent at detailing bony anatomy, and in the paranasal sinuses, bony changes of the skull base or lamina papyracea may differentiate polyps from other polyp-like masses. For example, focal hyperostosis seen adjacent to the edge of the polypoid mass may suggest the diagnosis of an inverted papilloma. Conversely, skull base erosion may indicate extrasinus expansion of a nasal polyp or mass, or the possibility of an encephalocele. In the setting of an isolated polyp-like mass with CT evidence of skull base erosion, magnetic resonance imaging is indicated to differentiate polyp from meningoencephalocele. CT scans can also be used for intraoperative navigation, especially in the setting of massive polyposis or revision surgery, where anatomy may be distorted.
Magnetic resonance imaging (MRI) is generally not performed in the workup of nasal polyposis, unless there are specific concerns of a meningoencephalocele or suspicion of a sinonasal tumor. MRI findings in allergic fungal sinusitis are characteristic, with a low T2 signal or signal void due to high concentration of various metals such as iron, magnesium, and manganese concentrated by fungal organisms as well as high protein and low free water content in allergic mucin. The inflamed mucosal lining will be hyperintense on T2-weighted imaging and demonstrate contrast enhancement (see Fig. 6.3a, b).
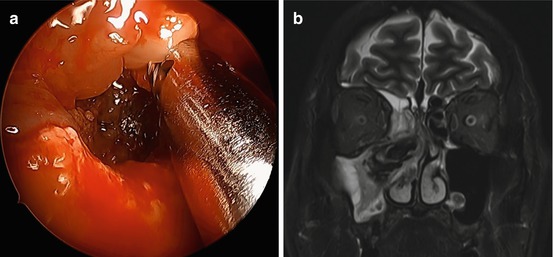

Fig. 6.3
(a) Allergic fungal sinusitis with polyps and fungal mucin obliterating the maxillary sinus. (b) T2-weighted MRI showing central hypointensity and signal void with peripheral hyperintensity in the inflamed mucosal lining
Allergy and Immune Workup
Allergy evaluation by skin or blood testing is indicated to rule out comorbid atopic disease that may play a role in some patients with CRSwNP. NSAID intolerance should also be specifically queried to rule out AERD. In patients with nasal polyposis who have had multiple recurring infections of the upper and lower respiratory tracts, a workup for selective immunodeficiency or common variable immunodeficiency may be considered, including measurements of serum immunoglobulin (Ig) levels and pre- and postpneumococcal vaccine titers. The possibility of Churg-Strauss syndrome should also be considered, especially in patients with preexisting asthma and allergic rhinitis with unexplained worsening of asthma and subsequent development of hypereosinophilia that may occur together with vasculitis. Cystic fibrosis (CF) testing should also be considered, either by sweat chloride test or genetic testing. Less commonly, primary ciliary dyskinesia may be present, and diagnosis will require obtaining nasal biopsy to examine ciliary ultrastructure by electron microscopy.
Treatment
The treatment of nasal polyposis has evolved, as an improved understanding of the underlying pathophysiological mechanisms has led to the development of newer therapies. In patients where surgery is contraindicated or not elected, medical therapy can be used to try to control or alleviate symptoms and polyp growth. Anti-inflammatory medications are playing a more important role now as there is a greater appreciation of the need to control inflammation and prevent polyp recurrence.
Surgical
Surgical treatment of nasal polyposis should not be viewed with an expectation to cure the disease, but rather as an important adjunctive therapy to ongoing medical treatment. Surgery can range from simple polypectomy to comprehensive endoscopic sinus surgery (ESS). Although the former may suffice in less severe disease as a temporizing measure, ESS with complete polyp removal and opening all paranasal sinuses should be considered the standard surgical therapy for symptomatic, medically refractory nasal polyposis. This not only reduces the inflammatory load and allows ventilation of the sinuses, but it also facilitates delivery of topical medications into the sinuses. Cadaveric and in vivo studies have shown that topical delivery of medications into the sinuses is improved post-ESS. Furthermore, a meta-analysis on topical steroid therapy for nasal polyps showed that the patients who underwent sinus surgery had a greater response to topical steroid treatments than those without surgery [39].
Certain surgical techniques may be employed to better optimize the surgical sinusotomies for delivery of topical medication. For example, partial resection of the middle turbinate has been shown to prolong the interval to revision surgery for nasal polyposis, although a retrospective study showed the time difference to be only 6 months [36]. The endoscopic maxillary mega-antrostomy, also known as modified endoscopic medial maxillectomy, can be helpful in the treatment of recalcitrant maxillary sinusitis [40]. This procedure involves removing the posterior half of the inferior turbinate and a portion of the medial wall of the maxillary sinus, so as to facilitate sinus hygiene and to improve delivery of topical medications and irrigations. This effect was possibly due to the increased air space which reduces polyp regrowth and allows better access for drug delivery.
Due to the propensity for recurrence of polyps, continued postoperative surveillance of the sinonasal cavities with serial endoscopy is important. For focal polyp recurrences, in-office polypectomies may help to maintain patency of sinus ostia for drug delivery without needing to bring the patient back to the operating room.
Medical
With newer methods of drug delivery, medical therapy has come to play an increased role in the management of nasal polyps. Whereas oral medications are useful to treat systemic conditions, for inflammatory disease restricted to the nose and paranasal sinuses, topical therapies can be directed to the target tissue while sparing systemic side effects.
Oral Steroids
Systemic corticosteroids can effectively reduce the size of nasal polyps, and while the benefits of relieving nasal obstruction and improving olfaction often outlast the duration of therapy, the therapeutic effects are relatively short-lived. For management of acute exacerbations and for pulsed steroid therapy, there is no evidence-based consensus on optimal dosing, although the upper limit in most studies has been 60 mg of prednisone daily for 20 days. Gastrointestinal upset, insomnia, and transient adrenal suppression are among the potential side effects of orally administered corticosteroids. When given preoperatively, oral steroids improve visualization of surgical field and potentially decrease blood loss and operative time. The literature suggests that a daily dose of 30 mg of prednisone started 5–7 days preoperatively is a safe and effective starting point [41]. Postoperatively, systemic steroids can help to reduce postsurgical edema and inflammation and prevent early synechiae formation [42].
While oral corticosteroids have been found to be effective in treating patients with classic eosinophilic polyps, patients with neutrophilic polyps seem to respond less favorably. Wen et al. found that oral steroid therapy reduces eosinophil counts but not neutrophil counts in nasal polyps, and patients with eosinophilic polyps had significantly better oral steroid responses than those with neutrophilic polyps, in terms of reduction of polyp size, nasal congestion and total nasal symptom scores, and nasal resistance [13]. Understanding the histopathology of nasal polyps may thus help to individualize therapy and improve treatment responses.
Topical Steroids: Sprays and Rinses
In contrast to the short-term efficacy of systemic steroids, the role of topical steroids is to maintain long-term control of nasal polyps. There are a variety of drug delivery mechanisms, ranging from low-volume low-pressure systems (nasal drops and sprays) to high-volume high-pressure systems (nasal irrigation). There is general agreement that topical steroid therapy helps to alleviate symptoms, reduces polyp size, and prevents recurrence of polyps postoperatively [43]. Intranasal corticosteroid sprays have been the most well studied and have proven to be efficacious for nasal polyposis.
Intranasal steroid irrigations have become popularized but are less well studied. The potential benefit of delivering a higher dose of corticosteroids locally while minimizing systemic side effects is attractive, although there are few randomized trials to date that specifically look at this therapy. Safety studies have found no negative effect on the hypothalamus-pituitary-adrenal (HPA) axis after 6–8 weeks of continuous irrigations with budesonide [44, 45].
Oral Antibiotics
Despite nasal polyposis being largely an inflammatory condition, infectious sinusitis may be an associated condition. Antibiotics can play a role in treating the infectious portion of the disease, although there are few randomized trials looking at this. Short-term courses of antibiotics should be considered, particularly when pus is present and can be cultured, as an adjunct to maximal medical therapy and in acute exacerbations. Antibiotic therapy longer than 3 weeks is generally not recommended for infectious indications.
Doxycycline and macrolide antibiotics have been used in the treatment of nasal polyposis for their intrinsic anti-inflammatory properties, which are thought to be more effective for neutrophil-associated inflammation than eosinophilic inflammation. Treatment duration is often longer than for infectious indications, even up to 1 year. One study compared doxycycline with methylprednisolone and placebo and found significant reduction in polyp size and postnasal drainage associated with doxycycline, although there was no other symptom improvement or improvement in peak nasal inspiratory flow (PNIF) [46].
Studies of macrolides have been characterized by heterogeneous inclusion criteria and treatment outcome measures. The number of high-quality studies is limited, with the majority being prospective observational studies. One controlled study showed improved patient symptoms and endoscopic findings using roxithromycin 150 mg daily for 3 months in refractory CRS patients, especially in the subgroup with low IgE levels [47], while another controlled study using low-dose azithromycin did not show any difference from placebo [48]. Overall data from observational studies however show general improvements in patient symptoms, endoscopic findings, imaging findings, and reduction of inflammatory markers within nasal mucus secretions [49]. Macrolides are thus a therapeutic option in the treatment of CRSwNP. Whether the beneficial effects are truly due to the anti-inflammatory properties or the antimicrobial effects of macrolide antibiotics, or a combination of both, bears further study. The optimal subgroup of patients who may benefit most from this therapy also remains to be determined.
Topical Antibiotics: Rinses
In contrast to topical corticosteroid therapy, topical antibiotic use in the management of nasal polyps is a lot more controversial. Theoretical benefits of topical antibiotics include eradication of biofilm and reduction of Staphylococcus superantigen load, although one small randomized trial showed only short-term improvement with mupirocin nasal rinses in recalcitrant Staphylococcal CRS which was not sustained in the longer term [50]. Studies have mostly focused on CRS and not specifically on nasal polyposis, so that it is not recommended for routine use in patients with CRSwNP.
Aspirin Desensitization
Aspirin desensitization is a key treatment modality in managing nasal polyposis in patients with AERD, as an adjunct to surgery and medical therapy. The exact mechanism of action is unknown, although rapid induction of oral tolerance is associated with a decrease in serum cysteinyl leukotrienes and improvement in the dysregulation of arachidonic acid metabolism [51, 52]. Aspirin desensitization has been shown to improve symptoms and quality of life, prevent polyp regrowth, and reduce the need for oral corticosteroids and revision surgery and is optimally initiated within 4–8 weeks after sinus surgery [53–56]. Desensitization protocols vary, although most can be executed in an ambulatory setting, in contrast to earlier years in which desensitization was performed in the intensive care unit. Once desensitization has been achieved, maintenance doses are typically from 650 mg to 1,300 mg daily for life, with repeat desensitization needed if more than 96 hours has elapsed between doses. Our experience with aspirin desensitization is that the majority of patients are able to tolerate and complete it, with sustained endoscopic and symptom improvement over a prolonged period [57].
Antifungal Therapy
Given the possible link between fungi and CRS, people have studied using antifungal therapy in the treatment of CRS and its subtypes. The results so far have not shown benefit for antifungal therapy in either CRSwNP or AFS. Two clinical trials evaluating nasal amphotericin B sprays in patients with nasal polyps did not show benefit in symptom scores [58, 59]. A more recent systematic review looking at antifungal therapy for AFS also showed no overall benefit in topical or oral antifungal therapy on endoscopic scores or patient reported outcomes, with no significant differences between treatment and control groups [60].
Immunotherapy
Immunotherapy should be considered in atopic patients with CRSwNP, as this can help with symptomatic outcomes postoperatively [61]. Fungal immunotherapy is also an option in treating patients with AFS specifically, since the disease is characterized by an excessive immune response to fungi. Studies are limited to using dilute antigen concentrations in patients who have been operated upon to decrease the inflammatory load, although recent practice parameters have advocated higher allergen concentrations [62]. Recommendations have also been made to initiate fungal immunotherapy 4–6 weeks after surgery, to avoid exacerbation of symptoms in patients with active AFS [63]. The overall role of immunotherapy can thus be seen as adjunctive in the treatment of nasal polyposis.
Leukotriene Modifiers
Leukotrienes are mediators in the inflammatory cascade, and so it is unsurprising that increased levels of leukotrienes and its receptors have been shown in nasal polyps. Leukotriene antagonists have been shown to be effective in chronic inflammatory conditions of the airway such as allergic rhinitis and asthma, conditions which are commonly coexistent with nasal polyposis. Examples of CysLT1 receptor antagonists include montelukast and zafirlukast, while zileuton is an inhibitor of 5-lipoxygenase. Randomized controlled trials have shown that montelukast is effective in symptom reduction of nasal polyposis compared with placebo and equally effective compared with intranasal corticosteroid sprays in this respect [64]. Combination therapy of montelukast with budesonide sprays showed significant improvement in headache, facial pain, and sneezing, compared to monotherapy with budesonide sprays [65]. Lower evidence studies have also shown zafirlukast and zileuton to provide similar symptom improvement in CRSwNP [66, 67].
When considering AERD where the underlying mechanism is due to dysregulation of arachidonic acid metabolism with decreased inhibition of the 5-lipoxygenase pathway, drugs such as the 5-lipoxygenase inhibitor zileuton should theoretically be effective in treating the disease. Ulualp et al. looked at zileuton and zafirlukast in AERD patients and found significant improvement in symptom scores, subjective reports, and endoscopic examination [66]. Montelukast was also found to have improvement in both aspirin-tolerant and aspirin-sensitive patients with nasal polyps and asthma, with improvements in polyp score only present in aspirin-tolerant patients [68]. Based on current literature, while we know that leukotriene antagonists can help in the management of nasal polyposis, finding the optimal use in the correct patient population and setting still requires further investigation.
Biologic Agents
The newest anti-inflammatory agents being investigated in the treatment of nasal polyposis are antibodies targeting parts of the inflammatory cascade. Anti-IL5 agents – mepolizumab and reslizumab – are humanized monoclonal antibodies that target eosinophilic inflammation. One trial evaluating reslizumab showed a decrease in total nasal polyp score and decrease in blood eosinophil counts compared to placebo, although there was rebound hypereosinophilia posttreatment [69]. There was however no significant difference in symptom scores or nasal peak inspiratory flow (nPIF) between treatment and placebo groups. Another trial compared mepolizumab against placebo and similarly showed improved nasal polyp score, blood eosinophil counts, and computed tomographic score [70]. These improvements were seen in 60 % of treated patients, and there were no markers to suggest possible responders to anti-IL5 therapy. There were also improvements in some symptom scores, namely hyposmia, congestion, and postnasal drip, although these did not reach significance.
Omalizumab is a recombinant DNA-derived humanized IgG monoclonal antibody that selectively binds to IgE. It has been studied in the treatment of asthma and allergic rhinitis and more recently in CRS. One trial specifically looked at the effect of omalizumab in treating patients with nasal polyps and comorbid asthma and found that there was significant reduction in total nasal polyp score with corresponding improvements in Lund-Mackay scores on CT [71]. There was also significant improvement in symptom scores and the Short-Form Health Questionnaire SF-36 on physical health, although no difference was found in mental health. No differences were found in treatment outcomes between allergic and nonallergic patients. There are however concerns with prescribing omalizumab, such as the risk of anaphylaxis in 1 patient per 1,000 as well as potential cardiac effects and thrombocytopenia. There is also the potential of higher than expected arterial thrombotic events. Earlier concerns regarding malignancies related to omalizumab seem unfounded, as recent studies do not suggest an association between omalizumab therapy and an increased risk of malignancy [72].







