Fig. 19.1
Acquired hypertrichosis lanuginosa. Reprinted from Current Problems in Surgery, 47, Ehst BD, et al. Cutaneous Manifestations of Internal Malignancy, 384–445, 2010, with permission from Elsevier
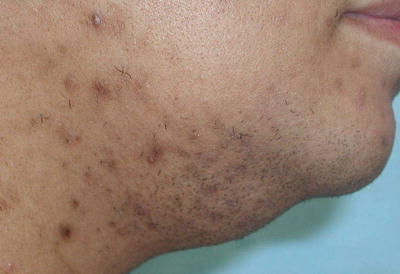
Fig. 19.2
The development of course terminal hairs in a male pattern, in a woman as seen here, is classic for hirsutism. Courtesy of Julia R. Nunley, M.D.
Differential Diagnosis [3]
Acquired Generalized Hypertrichosis:
Medications
Malnutrition
Anorexia nervosa
Head injury
Hepatic porphyria
Hypothyroidism
Dermatomyositis
AIDS
Paraneoplastic hypertrichosis
Workup [13]
An examination of a patient with hypertrichosis should include the following: type of hair involved (e.g., lanugo, vellus, terminal), hair growth pattern, age of onset, history of present or past systemic disorders, medication history, family history, and ethnic and racial background. It must be always kept in mind that hypertrichosis may be a manifestation of a more general medical problem.
Treatment
Hypertrichosis caused by medications, including cyclosporine, is reversible upon discontinuation of the offending agent within several months to 1 year depending on the hair cycling characteristics of the affected site [3]. In the transplant setting, the substitution of a cyclosporine-based regimen with a tacrolimus-based regimen was found to significantly improve hair growth and patient’s quality of life without increasing the risk of renal allograft dysfunction or rejection [14–16]. The discontinuation or substitution of cyclosporine with an alternative immunosuppressant should first be discussed with the patient’s transplant provider.
In addition, concomitant use of cyclosporine and minoxidil has been reported to augment hypertrichosis and therefore alternate therapy should be considered [17].
There are a number of treatment options for excess hair growth that can be categorized into depilatory or epilatory methods. Depilation is the removal of hair at some point along its shaft, whereas epilation is a process that removes the entire hair shaft. Epilation methods last longer than depilatory methods and may even cause sufficient damage to the follicle to provide long-term or permanent hair removal; however, only destruction of the follicular germ cell will produce permanent hair removal. Individual patient preferences play a role in selecting the most appropriate therapy [3, 18, 19].
Mechanical and chemical depilatory methods are convenient and most widely used, but must repeated often for adequate management of excess hair. Shaving is fast, safe, and effective, but this method is not generally well accepted by adult women for facial hair. Using a pumice stone in the affected area may help remove fine hairs, but can cause irritation and dermatitis with inappropriate use. Two forms of chemical depilatories are available: sulfides of alkali metals and thioglycolate salts. These agents dissolve hair shafts by breaking disulfide bonds. The sulfide preparations are more effective but also more irritating to the skin. Chemical depilatories should be tested on a small area of the skin before widespread use [3, 18, 19].
Epilation, removes hairs down to the hair bulb, and includes tweezing, waxing, and threading. Tweezing or wax epilation of an affected area usually produces good results by removing hairs with the root. Threading is a common technique used in the Middle East which involves a twisted string run rapidly over a hair-bearing area, removing hairs along with it. All of these techniques can cause mild to moderate pain [3, 18, 19].
More permanent methods of hair removal include electrolysis, laser epilation, and photoepilation. Electrolysis, either galvanic or thermal, is painful and time-consuming because each hair follicle needs to be individually targeted. For this reason, electrolysis is a good option only for treating small areas of skin. Laser therapy, which is more expensive, is less painful, faster, and more effective than electrolysis. However, a recent Cochrane review of hair removal methods found little evidence of their effectiveness [20]. Alexandrite and diode lasers reduced hair by approximately 50% up to 6 months after treatment. Less evidence is available for short-term effects of pulsed light, neodymium:yttrium-aluminum-garnet (Nd:YAG), and ruby lasers, and none of these treatments have well-documented long-term outcomes. Laser therapy works best on dark hair but posttreatment hyperpigmentation may occur.
Since most drug-mediated cases of hypertrichosis are not androgen-mediated, antiandrogen therapies may be minimally helpful. Eflornithine (Vaniqa™) is a topical agent that reduces hair growth through inhibition of the enzyme ornithine decarboxylase that is present in hair follicles and important in hair growth. When used for excess facial hair, results are noticed in about 8 weeks. Eflornithine can be used alone or in conjunction with other therapies; side effects include stinging, burning, tingling, and erythema. Hair growth resumes upon discontinuation of eflornithine [21].
Gingival Hyperplasia
Definition/Physiology/Pathogenesis
Gingival hyperplasia is an increase in the size, or growth, of the gingiva. Overgrowth of the gingiva can be caused by a number of factors, including inflammatory conditions and as a side effect of certain medications. Gingival hyperplasia caused by medications is often referred to as drug-induced gingival overgrowth (GO) or gingival enlargement [22]. The use of cyclosporine and calcium channel blockers such as nifedipine, verapamil, amlodipine, and anticonvulsants have been reported to contribute to GO [23].
Though the pathophysiology of drug-induced GO is not completely understood, it is believed to be multifactorial. Studies have shown that the interaction of phenytoin, cyclosporine, and nifedipine with epithelial keratinocytes, fibroblasts, and collagen can lead to GO in susceptible individuals. Both genetic and cellular mechanisms of cyclosporine GO have been proposed. Studies suggest that cyclosporine increases the number of gingival fibroblasts; on the contrary other studies suggest that cyclosporine increases interleukin (IL)-6, a cytokine that inhibits fibroblast proliferation. In fact, IL-6 is known to enhance collagen and glycosaminoglycan synthesis [24, 25]. Additionally, fibroblasts from cyclosporine GO have shown reduced phagocytic activity; therefore an increased rate of synthesis paired with a decreased rate of phagocytosis could result in the increase in connective tissue volume [26]. Recent studies suggest that genetic polymorphisms may also be involved in determining susceptibility to GO caused by this medication [27, 28].
A variety of risk factors may also contribute to the severity of GO caused by cyclosporine. The presence of gingivitis, or gingival inflammation, resulting from poor oral hygiene is a significant factor and can exacerbate the effect of medications [22]. Children and teenagers on cyclosporine are at an increased risk, which suggests a hormonal component. In this population there are higher circulating levels of androgens, and this can have a stimulating effect on gingival fibroblasts to increase collagen synthesis [29]. Males are three times more likely to develop overgrowth compared to females [22]. The relationship between cyclosporine serum concentrations, dosage, and gingival enlargement is controversial. A variety of pharmacokinetic parameters have been investigated with inconsistent findings and, as such, are considered poor prognostic indictors for GO. In spite of these shortcomings, there is a general agreement that an initial, unidentified, threshold serum concentration is required to initiate the overgrowth process [29, 30]. Frequently, patients on cyclosporine are also on concomitant calcium channel blocker therapy that increases the prevalence of GO significantly [30].
Immunosuppressants Associated with Gingival Hyperplasia
Clinical Features
The onset of cyclosporine GO generally appears within the first 3 months of drug administration [31]. The growth starts in the interdental papillae and extends to the facial and lingual aspects of the gingival margin, as seen in Fig. 19.3 [32]. The appearance of GO in patients on cyclosporine often presents with a more vascularized, lobulated, inflamed gingiva that bleeds easily compared to GO caused by other medications [33]. The enlargement may become a massive amount of tissue that covers the crowns of the teeth and can interfere with mastication, speech, esthetics, and can lead to shifting of teeth and malocclusion. Gingival enlargement can also complicate the patient’s oral hygiene and the patient ability to clean the teeth, thus increasing the inflammatory process and in return further increasing GO [22].
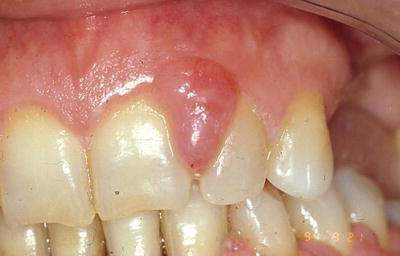

Fig. 19.3
Gingival hyperplasia
Differential Diagnosis [13]
Generalized gingivitis
Pregnancy gingivitis
Puberty gingivitis
Pyogenic granuloma
Leukemia
Workup [34]
Periapical or panorex radiographs are indicated prior to treatment to evaluate the status of the periodontal tissue or any compromised teeth. Complete blood count (CBC) with platelet count is indicated in patients with severe gum bleeding to rule out anemia and leukemia. Culture is recommended to rule out oral candidiasis. Tissue biopsy may be indicated if GO has an unusual clinical presentation or if the patient is not on a medication known to induce GO.
Treatment
Oral hygiene and plaque control combined with the removal of local factors are essential for any patient taking drugs associated with gingival enlargement. While excellent oral hygiene and professional plaque control can potentially prevent or lessen the severity of the condition, they often are insufficient for reversing the process once established [23].
The most effective treatment is the withdrawal of the causative medication and substitution with others. Evidence suggests that GO may resolve in 1–8 weeks in some patients with drug substitution or withdrawal [35]. In the transplant setting, the substitution of a cyclosporine-based regimen with a tacrolimus-based regimen was found to significantly improve gingival hyperplasia without increasing the risk of renal allograft dysfunction or rejection [16]. The discontinuation or substitution of cyclosporine with an alternative immunosuppressant should first be discussed with the patient’s transplant provider.
If cyclosporine substitution is not an option, case reports suggest that the gingival hyperplasia can be effectively treated with a 2-week course of metronidazole (750 mg three times per day) while cyclosporine is continued [36, 37]. It is not clear if metronidazole acts in this setting via its antibacterial activity or via another mechanism. Treatment with azithromycin (500 mg per day for 3 consecutive days) may also be effective, particularly among those with mild or early disease [38].
While nonsurgical therapy and, if possible, drug substitutions should be attempted first, surgical or laser gingivectomy may be required. However if the offending agent is not discontinued, recurrence of GO can ensue, requiring additional surgical procedures [39].
Sebaceous Gland Hyperplasia
Definition/Physiology/Pathogenesis
Sebaceous hyperplasia (SH) is an enlargement of sebaceous glands surrounding a follicle.
In general SH is a well-recognized benign condition in older adults. The causative factors include intrinsic and photoaging, accompanied by reduced androgen levels leading to decreased cellular turnover in sebaceous glands, resulting in hyperplasia [40, 41].
Sebaceous hyperplasia is also possible in other populations, though less common. Premature or familial cases of SH have been reported in younger individuals, suggesting a genetic predisposition [42–44]. Muir-Torre syndrome (MTS) is a rare autosomal dominant disorder in which sebaceous neoplasms have been reported in association with a visceral malignancy, usually gastrointestinal or genitourinary carcinomas [45]. Sebaceous hyperplasia has also been linked to transplant recipients taking cyclosporine, though the mechanism is poorly understood. Since cyclosporine is highly lipophilic, it has been suggested that the sebaceous gland may be the major site of cutaneous accumulation leading to hyperproliferation and arrest of sebocytes causing SH. Genetics and hormones may also influence the development of SH caused by cyclosporine as case reports only include male patients [41, 46, 47].
Immunosuppressants Associated with Sebaceous Hyperplasia
Sebaceous hyperplasia is a common skin finding in aging adults, reported to occur in approximately 1 % of the healthy population. In case reports, however, the prevalence of SH has been reported to be as high as 10–16 % in patients receiving long-term immunosuppression with cyclosporine [46, 48, 49].
Clinical Presentation
Sebaceous hyperplasia consists of asymptomatic, small flesh-colored to yellow papules with central depression from which a very small amount of sebum can sometimes be expressed, as seen in Fig. 19.4 [50]. The nose, cheeks, and forehead are primarily affected; however very rarely SH can occur on the chest, areola, mouth, scrotum, foreskin, penile shaft, and vulva. Sebaceous hyperplasia appears at higher frequencies after 40–50 years of age and increases in prevalence over time. It is important to note that SH alone does not signify a predisposition to cancer or represent a sign of MTS [40, 51–54].
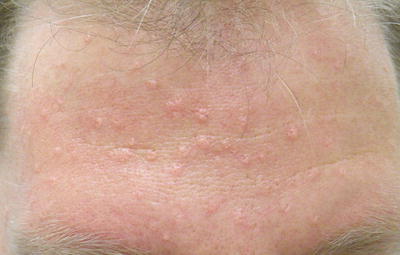

Fig. 19.4
Sebaceous hyperplasia. Courtesy of Julia R. Nunley, M.D.
Cyclosporine can induce SH over an expansive time period, with a clinical presentation ranging from 3 to 19 years of cyclosporine use. In case reports those patients with cyclosporine-induced SH were all male, suggesting a possible genetic and hormonal basis to the development of SH after organ transplant [41, 42, 47]. A recent study found 45.7 % of renal transplant patients with SH to have a history of nonmelanoma skin cancer compared to only 7.3 % of patients without SH. This strong association of nonmelanoma skin cancer with SH remained significant after correction of factors such as age, sex, skin type, and duration since the transplantation [55].
Workup
Dermoscopy may be useful as a noninvasive tool to aid in the clinical diagnosis and in distinguishing between nodular basal cell carcinoma and sebaceous hyperplasia, reducing unnecessary surgery [56].
Treatment
Sebaceous hyperplasia is completely benign and does not require treatment; however, lesions can be cosmetically bothersome. Treatments are mostly mechanical. Lesions tend to recur unless the entire unit is destroyed or excised. Risk of permanent scarring must be considered when treating benign lesions. The following therapies have been reported to be somewhat effective: photodynamic therapy, cryotherapy, electrodessication, topical chemical treatments (e.g., bichloracetic or trichloroacetic acid), laser treatment (e.g., argon, carbon dioxide, pulse-dye laser), shave excision, and excision [57–60].
Oral isotretinoin can be effective for the treatment of SH because of its ability to temporarily shrink sebaceous glands. Patients with diffuse multiple lesions, including those on cyclosporine therapy, had clearing after 2–6 weeks of treatment. Doses of 10–40 mg every other day can be used. Upon discontinuation of therapy, SH lesions will reoccur. oral isotretinoin should be taken with extreme caution; this medication is pregnancy category X and is known to cause major fetal abnormalities [43, 46, 61]. In the United States access to this medication is restricted. All patients (male and female), prescribers, wholesalers, and dispensing pharmacists must register and be active in the iPLEDGE™ risk management program, designed to eliminate fetal exposures to isotretinoin. This program covers all isotretinoin products (brand and generic). The iPLEDGE™ program requires that all patients meet qualification criteria and monthly program requirements (e.g., pregnancy testing). Healthcare providers can only prescribe a maximum 30-day supply at each monthly visit and must counsel patients on the iPLEDGE™ program requirements and confirm counseling via the iPLEDGE™ automated system [62].
Acneiform Eruptions/Acne
Definition/Physiology/Pathogenesis
Acne is one of the most common pustular skin conditions [63]. Androgen production after puberty stimulates the release of sebum by the sebaceous glands; if the flow of sebum is impeded due to abnormal keratinization in the pilosebaceous canal, it can lead to the formation of comedones [2, 63]. Inflammation, bacterial overgrowth or infectioncan result in papules, pustules, and cysts [63, 64].
Immunosuppressants Associated with Acne
Acne is reported in 2–15 % of organ transplant recipients [66]. It is usually associated with steroid and cyclosporine use; however, it has been reported with several of the immunosuppressants [66]. In a study of 80 patients, the frequency of acne in prednisolone and cyclosporine-treated patients was reported to be 36 % [67].
In sirolimus trials the frequency of acne is estimated to be 15–25 % and is listed at 22 % in the package insert [66, 68]. In a study of cutaneous adverse events in 80 renal transplant patients receiving sirolimus-based therapy, 46 % of patients experienced acne-like eruptions [66]. The skin eruptions occurred soon after initiating sirolimus (median: 1 month); they were more common in men than women, possibly suggesting a hormonal role for this adverse effect [66].
Everolimus has been reported to cause acne in ≥1 to <10 % of kidney and liver transplant patients; however, data in kidney transplant patients includes everolimus in combination with basiliximab, cyclosporine (at reduced doses), and corticosteroids [69]. In a study of everolimus versus placebo in autosomal dominant polycystic kidney disease, acne was reported in 14 % of patients with everolimus compared to 2.8 % of placebo patients [70]. Compared to placebo, basiliximab-treated patients experienced ≥10 % of skin adverse effects, including acne [71].
Two randomized, active-controlled 3-year trials of belatacept in de novo kidney transplant patients where belatacept was used in combination with basiliximab induction, mycophenolate mofetil, and corticosteroids reported an 8 % incidence of acne [72




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








