Disorders affecting serum calcium and phosphate levels and parathyroid hormone secretion
Disorder or medication
Effect of disorder or medication
Serum calcium
Serum phosphate
PTH production
Hyperparathyroidism
Primary
PTH-secreting tumors (adenoma, multiple endocrine neoplasia syndrome)
↑
↓
↑
Secondary
Excessive PTH secretion caused by hypocalcemia
↑
↑
↑
Tertiary
Secondary hyperparathyroidism and PTH-secreting tumor
↑
↑
↑
Hypoparathyroidism
After parathyroidectomy, residual glands are suppressed from prior hyperparathyroidism
↓
↑
↓
Gland destruction caused by heavy metal (copper, iron) toxicity, granuloma, autoimmune disorder
↓
↑
↓
Magnesium deficiency (PTH secretion depends on normal magnesium levels)
↓
↑
↓
Renal failure
Decreased renal phosphate excretion results in hypocalcemia and secondary hyperparathyroidism
↓
↑
↑
Tumors
PTH is produced by parathyroid adenoma, other tumors
↑
↓
↑
PTHrP is produced by breast cancer, squamous cell carcinoma, lymphoma, myeloma
↑
↓
nl or ↓
Caused by tumor secretion of cytokines and PTHrP
↑
↓
nl or ↓
Vitamin D is produced by lymphomas
↑
↑
nl or ↓
Hypovitaminosis D
Decreased calcium and phosphate absorption from the GI tract and decreased resorption from bone and kidney results in hyperparathyroidism
↓
↓
↑
Aluminum toxicity
Decreased bone responsiveness to vitamin D and PTH
↑
↑
↓
Granulomatous disease
Synthesis of vitamin D2 by granuloma (sarcoidosis, tuberculosis, lymphoma-related granuloma)
↑
↑
↓
The Role of Anti-mineralization Proteins
Fetuin-A (α2-Heremans-Schmid glycoprotein) is a member of the superfamily of cysteine protease inhibitors. Unlike local inhibitors of calcification, fetuin-A is a ubiquitous serum protein that inhibits calcification at a systemic level by sequestrating circulating calcium and phosphate into colloidal spheres [60]. Mice lacking fetuin-A, although phenotypically normal, developed diffuse STC when fed a vitamin D-rich diet (12897203). In CKD patients, chronic inflammation may promote the development of calciphylaxis via reduction of fetuin-A levels [12]. Clearance of serum fetuin–mineral complex resulting in low serum fetuin-A levels may underlie vitamin D-induced vascular calcification [61]. In an cross-sectional study of 312 stable hemodialysis patients, low levels of fetuin-A were associated with increased cardiovascular mortality [62]. Low fetuin-A levels may also mediate STC in the absence of renal impairment [63].
Another strong inhibitor of vascular calcification is the vitamin K-dependent matrix Gla protein (MGP). This protein is synthesized by chondrocytes, VSMCs, endothelial cells, and fibroblasts [64]. Gamma-carboxylation is necessary for its activation [65]. The mechanisms whereby MGP inhibits vascular mineralization are multifarious including inhibition of calcium–phosphate precipitation and neutralization of bone morphogenic protein (BMP)-2-mediated calcification [66]. BMP-2 is implicated in transition of VSMCs to osteoclast-like cells, a crucial step in the development of vascular calcification. Recently, it has been shown that hyperphosphatemia-induced vascular calcification may be abolished by the calcimimetic calindol via upregulation of MGP synthesis [67].
Thrombosis
An under-recognized aspect of the observed entity of calciphylaxis is thrombosis of the calcified vessels—Mayo Clinic researchers feel this is fundamental to the observed mortality and morbidity. After all, calcification of blood vessel walls alone occurs in many disease processes such as diabetes, but often does not lead to morbidity. In calciphylaxis, it is thrombosis of the blood vessels that is the “tipping point”—leading to skin ulceration, which becomes infected, patients become septic and die. Weenig et al. reported in the Mayo Clinic series that 80 % of skin biopsies in calciphylaxis demonstrate thrombosis. He theorized that the only reason the figure was not 100 % was sampling on skin biopsy.
Why thrombosis? The answer is unknown, but several factors can be considered. First, the calcification of the blood vessels can act as a nidus for thrombosis (analogous to atherosclerosis in the coronary arteries). Second, patients with renal failure and other underlying conditions may be in a pro-thrombotic state-indeed preliminary studies, though few, have demonstrated low protein C and S, or antiphospholipid syndrome.
Clinical Features
Calciphylaxis presents with a spectrum of skin lesions ranging from livedo reticularis- or racemosa-like pattern to deep ulcerations. Very early changes may be clinically imperceptible or manifest as patchy areas with a net-like vascular pattern (livedo reticularis exquisitely tender, indurated) or a broken and branching morphology (livedo racemosa). At this stage, the histopathological correlate is the presence of cutaneous arteriolar stenosis due to intimal fibrosis and media calcification, leading to narrowing of the vessel lumen and reduced blood. When true vascular thrombosis with complete luminal obliteration ensues, purpuric-like lesions and deep and excruciatingly painful ulcerations and subcutaneous nodules appear. They usually retain their retiform configuration, a clue towards the correct diagnosis for the trained eye. Moreover, the same net-like blood vessel calcification configuration can be appreciated in soft-tissue radiographs and may serve as an additional diagnostic tool with a specificity approaching 90 % [68]. Not infrequently, however, they may adopt bizarre shapes with overlying necrotic eschars and dry gangrene.
In terms of distribution, lesions favor areas of high adiposity such as the abdomen, thighs, and buttocks [8, 69, 70] (Fig. 10.1). The head and neck are usually spared. Proximal necrotic lesions (trunk, buttocks, and thighs) are associated with higher mortality as compared to distal (below the knee) and acral disease [71].
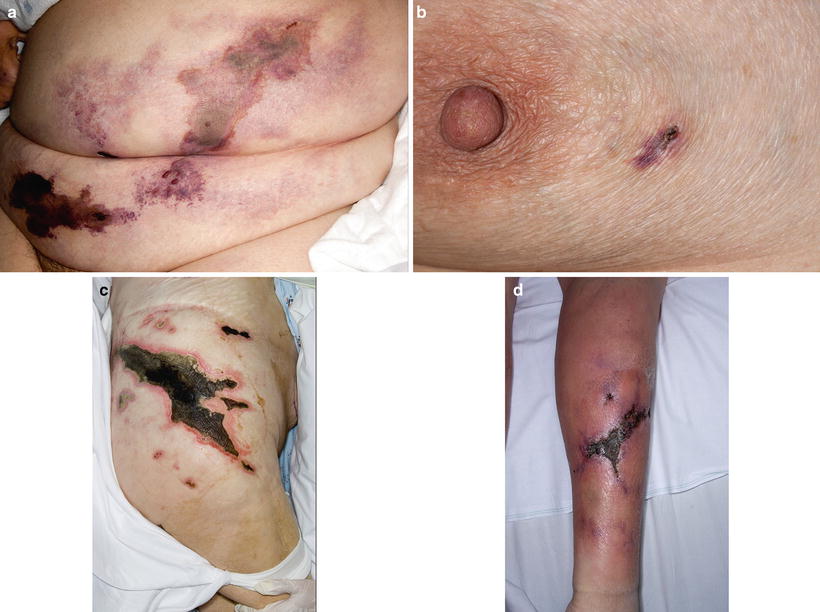

Fig. 10.1
(a) Characteristic retiform purpura on the abdomen. (b) Small purpuric lesion on the left lateral breast. Note incipient ulceration on the superior aspect. (c) Extensive involvement of the left flank and upper thigh with deep ulceration and eschar formation with smaller satellite lesions. (d) Involvement of the leg. Note edema and erythema extending beyond the central stellate ulcer
Prognosis
The prognosis of calciphylaxis is ominous with mortality rates approaching 46 % at 1 year and 80 % at 2 years from diagnosis [47]. The presence of ulceration portends a bad prognosis with a twofold increase in mortality [72]. Another study documented that 1-year survival rate is about 45 versus 35 % at 5 years [73]. For patients undergoing surgical debridement the 1-year survival rate is nearly 62 % [47].
Postmortem Findings
Few studies have been published. Intriguingly however, a study of three patients with calciphylaxis who had postmortem evaluation at Mayo Clinic demonstrated that calciphylaxis only involved the skin: there was no evidence of a similar process internally. The reason for these findings is unclear [74].
Histopathology
Multiple entities can mimic calciphylaxis. Therefore, for definitive diagnosis a deep skin biopsy, including sampling of the subcutaneous tissue, is needed. Histopathologic findings vary depending on the age of the lesion. Early lesions may show only subtle changes. Late lesions are characterized by dermal necrosis and intramural calcium deposition of dermal and pannicular vessels [75] as well as vascular thrombosis and endovascular fibroblastic proliferation [47, 75] (Fig. 10.2). The most common finding overall is the presence of calcifying septal panniculitis [75]. More recently, it has been suggested that when typical features of vascular and extravascular calcification are not evident, the presence of perieccrine calcification may point towards the correct diagnosis [76]. Fragmented and calcified elastic fibers as seen in pseudoxanthoma elasticum may also be a histopathologic finding [77]. A von Kossa stain highlights calcium deposits in the vascular walls and in collagenous septa of subcutaneous tissue [78].
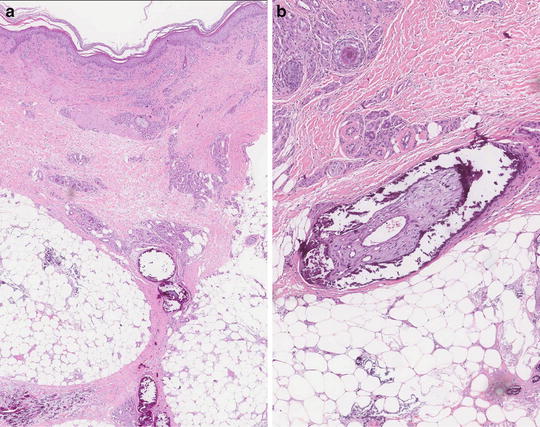

Fig. 10.2
(a) Lobular pannicular necrosis with calcified and thrombosed pannicular vessels and mixed lobular pannicular inflammation. (b) Higher magnification showing pannicular vessel calcification and thrombosis
Differential Diagnosis
Fully developed lesions of calciphylaxis have a distinct clinical appearance referred to as retiform purpura. A number of cutaneous disorders share a similar morphology and should be considered in the differential diagnosis (Table 10.2). A thorough history, careful examination of the skin lesions, risk factor analysis, and histopathologic evaluation will render the correct diagnosis. In the right clinical setting, visual recognition alone will be highly suggestive but incisional biopsy (to include subcutaneous fat) is necessary for confirmatory purposes.
Table 10.2
Differential diagnosis of calciphylaxis based on clinical morphology. Most common conditions that present with “retiform purpura”
Differential diagnosis of calciphylaxis based on clinical morphology. Most common conditions that present with “retiform purpura” |
|---|
– Vasculitis (Wegener granulomatosis, polyarteritis nodosa, Churg–Strauss syndrome, microscopic polyangiitis) |
– Antiphospholipid syndrome, lupus anticoagulant, protein C and S dysfunction, heparin necrosis, warfarin necrosis |
– Cold-related vascular occlusion (cryoglobulinemia, cryofibrinogenemia) |
– Cutaneous anthrax, ecthyma gangrenosum, disseminated strongyloidiasis, mucormycosis |
– Panniculitis |
– Purpura fulminans |
– Pyoderma gangrenosum |
– Livedoid vasculopathy |
– Thromboangiitis obliterans |
Associated Conditions and Risk Factors
ESRD is the most important and widely recognized condition associated with calciphylaxis [47, 79]. Within this patient population several risk factors have been linked to an increased risk for development of calciphylaxis including: white race [49], liver disease, systemic steroid use, CPP greater than 70 mg2/dL2, serum aluminum more than 25 ng/mL, obesity [47], female gender, peritoneal dialysis, diabetes mellitus, concurrent therapy with calcium salts and vitamin D [50, 80], increased serum phosphate levels, elevated alkaline phosphatase [73], warfarin therapy, poor nutritional status [48], and increased sedimentation rate [81].
Non-uremic calciphylaxis cases have also been reported in patients with normal renal and parathyroid function and in association with connective tissue disorders (systemic lupus erythematosus, rheumatoid arthritis, giant cell arteritis), hematologic malignancies (multiple myeloma, chronic myelocytic leukemia), diabetes (without concomitant renal failure), primary hyperparathyroidism, vitamin D deficiency, protein C and S deficiency, warfarin therapy, factor V Leiden deficiency, Crohn disease, primary autoimmune myelofibrosis, and liver disease [14, 46, 49, 53, 82–88]. It is expected that this list will continue to expand with increased recognition of this condition.
Evaluation
Table 10.3 lists a recommended initial workup for patients in whom calciphylaxis is suspected. In the authors’ opinion a deep skin, preferentially excisional, biopsy to include subcutaneous tissue is of utmost importance in establishing the diagnosis.
Table 10.3
Suggested evaluation for patients with suspected calciphylaxis
Suggested evaluation for patients with suspected cutaneous calciphylaxis |
|---|
– Detailed history including past and current medications (warfarin, calcium and vitamin D supplements, phosphate binders, systemic steroids) |
– Liver enzymes, alkaline phosphatase, and bilirubin |
– Erythrocyte Sedimentation rate |
– Serum glucose |
– Serum creatinine and BUN |
– Serum calcium, phosphorus and PTH |
– Coagulation studies: prothrombin time, protein C and S levels, lupus anticoagulant, anticardiolipin, anti β2-glycoprotein; consider more comprehensive hypercoagulable state testing |
– Nutritional status assessment: body mass index, serum albumin, vitamin D levels |
– Skin biopsy: Deep, to include subcutaneous tissue |
– Plain soft-tissue radiographs to look for netlike pattern of calcification |
– In non-uremic patients and based on clinical suspicion: Consider serum protein electrophoresis, special coagulation studies (factor V Leiden, homocysteine, etc.), antinuclear antibodies, and rheumatoid factor |
Treatment
Management of calciphylaxis is challenging; multiple authors now suggest that multidisciplinary collaboration is essential. The main goals of treatment are identification and correction of underlying metabolic derangements, addressing vessel thrombosis that is leading to the ulcerations, arresting disease progression, wound care, pain control, and prevention of gangrene formation and fatal sepsis. To this end, aggressive wound care are of utmost importance.
In practice, efforts must be focused additionally on pain control, symptomatic management, and palliative care.
Renal Replacement Therapy and Normalization of Metabolic Disturbances
Traditionally, the standard of care for calciphylaxis has focused on restoration of the calcium–phosphate homeostasis by intensified hemodialysis [53, 89], restriction of calcium intake [90], low-calcium dialysates [91, 92], use of non-calcium-containing phosphate binders and correction of secondary hyperparathyroidism via calcimimetics or surgical parathyroidectomy when indicated.
Cinacalcet enhances the sensitivity of the calcium sensing receptor to extracellular calcium, thereby reducing serum calcium and PTH levels and normalizing secondary hyperparathyroidism. Paracalcitriol is a synthetic analog of calcitriol capable of selectively activating vitamin D receptors and, therefore, vitamin D responsive pathways, which in turn results in inhibition of PTH secretion. These calcimimetics have been reported to be valuable when use as part of a multimodal approach [93–96].
Parathyroidectomy has the potential advantage of eliminating excess PTH, thus normalizing calcium–phosphate levels. Unfortunately, the results are rather conflicting with some studies documenting improvement in wound healing and survival [97, 98], whereas others reporting no statistical difference [47, 99]. Importantly, it is not an innocuous procedure and complications may arise including poor wound healing, infection and metabolic alterations, to name a few [100]. Notably, complete resolution of severe calciphylaxis has been reported following kidney transplantation, but only in some case reports [101].
Bisphosphonates increase OPG production, thereby decreasing extraosseous mineralization [111]. In a case series of eight patients with calciphylaxis, administration of bisphosphonates resulted in rapid pain control and arrest of disease progression as soon as 2 weeks into treatment with no recurrence documented up to 9 years upon cessation of treatment [112]. Contrary to this observation, a patient with parathyroid adenoma developed calciphylaxis 9 months into treatment with bisphosphonates [113].
Sodium Thiosulfate
Since its first successful use for the treatment of calciphylaxis in 2004 (15168392), a beneficial role for sodium thiosulfate (STS) intravenously or by intraperitoneal infusion [102] has been increasingly documented in case reports and some case series. STS is a potent antioxidant and may improve the endothelial dysfunction associated with calciphylaxis [103]. It is also a chelator that binds calcium to form highly soluble calcium thiosulfate salts that are easily excreted by the kidneys or extracted by hemodialysis thereby improving wall vessel calcification. There are reports on its use in both uremic [104, 105] and non-uremic forms of calciphylaxis [106, 107]. It is generally well tolerated but its administration can be complicated with the development of non-anion gap metabolic acidosis [108]. However, other studies have failed to demonstrate improve survival.
Management of Thrombosis
Thrombosis is the final common pathway which results in tissue necrosis, infarctive skin ulcerations and consequent pain, morbidity and mortality. Therefore, thrombolysis and prevention of future thrombosis are essential management goals.
Hypercoagulability may contribute to the development of the disease [109, 110]; thus, it is conceivable to hypothesize that restoration of blood flow via thrombolysis would halt disease progression. A case report from Mayo Clinic researchers showed marked improvement in cutaneous ulcers and pain with administration of tissue plasminogen activator (tPA) [9]. This observation was further supported by a retrospective study of 15 patients that demonstrated that daily low-dose of tPA may be useful when used in conjunction with other therapies [8]. However, a mortality benefit has not been documented to date.
Wound Care
As with other treatment modalities, conflicting data and opinions exist regarding wound debridement in the management of calciphylactic ulcers. Experience at the Mayo Clinic documented that surgical debridement was associated with 1-year survival of approximately 62 % compared with only 27 % in patients who did not receive such intervention [47]. In a different study of 26 patients, surgical debridement was also associated with improved survival [97]. Aggressive wound debridement followed by split-skin transplantation has also been advocated [114]. Hyperbaric oxygen therapy has emerged as an adjunct in the management of calciphylactic ulcers. It enhances wound healing when delivered in combination with other therapies [115].
Pain Control and Palliative Care
Given the poor prognosis and severe pain associated with calciphylaxis, pain control and palliative care should actively be involved in the care of these patients to ensure mitigation of their discomfort and improvement of their quality of life [116].








