Fig. 18.1
Algorithm for recommended follow-up for patients with chronic kidney disease
Table 18.1
Recommended follow-up intervals for full-body skin exams for renal transplant recipients
Risk factors | Interval between full-body skin exams (no. of months) |
|---|---|
Field damage only | 6 |
Warts only | 6 |
One “low-risk” NMSC | 6 |
Field damage plus 1 NMSC in past 2 years | 3 |
History of 2 NMSC in past 2 years | 3 |
One NMSC (>2 cm) diameter on any anatomic location | 3 |
NMSC occurring in chronic wound or site of prior radiation | 3 |
One NMSC skin cancer with: –Moderately/poorly differentiated histology –Acantholytic histology | 3 |
One NMSC with perineural invasion | 3 |
Diagnosis
At times it can be difficult to distinguish nonmalignant lesions from malignant lesions by visual inspection alone in transplant recipients. Transplant patients frequently develop multiple hyperkeratotic, HPV-related papules that are visually indistinguishable from cutaneous malignancy. Neurological symptoms, specifically pain, may be an indicator for perineural invasion (PNInv) and should prompt concern for a cutaneous SCC [62]. These lesions always warrant a biopsy.
Despite the advent of new devices including the dermatoscope (magnifying glass with polarized light) and digital image analysis systems to aid visual inspection by the clinician, tissue evaluation after biopsy remains the gold standard for the diagnosis of skin cancers.
Actinic Keratosis (AK)
AKs are rough scaly pink spots that are typically seen on sun-exposed areas. A sandpaper-like texture allows them to be more easily palpated than seen. They are considered premalignant lesions and if left untreated, 6–25 % may evolve into SCCs [63–65]. It has been suggested that HPV infection may contribute to AK development in RTRs as a co-carcinogen, with UV exposure (see Fig. 18.2).
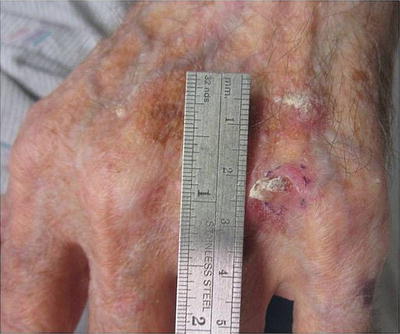

Fig. 18.2
Actinic keratoses (AKs). Two pink papules with sandpaper-like texture: a more sessile lesion proximally and one with overlying cutaneous horn distally. Both morphologies are consistent with actinic keratoses (AKs). Actinic keratoses are premalignant lesions caused by UV damage. If left untreated, they may transform into squamous cell carcinomas (SCCs). Aggressive management of AKs in renal transplant patients is necessary to minimize the development of SCCs
Warts
Warts represent cutaneous HPV infection, which some consider to be premalignant lesions in the setting of immunosuppression due to the potential for HPV-mediated malignant transformation [66]. They may appear as small, rough pink or whitish growths (verruca vulgaris) or flat, smooth lesions (verruca plana) (see Fig. 18.3). In immunosuppressed patients, warts can become large, keratotic, painful nodules and incur significant morbidity [67]. It can be difficult to tell nonmalignant warts from SCCs; a biopsy is recommended for any suspicious verrucous lesion (see Fig. 18.4).
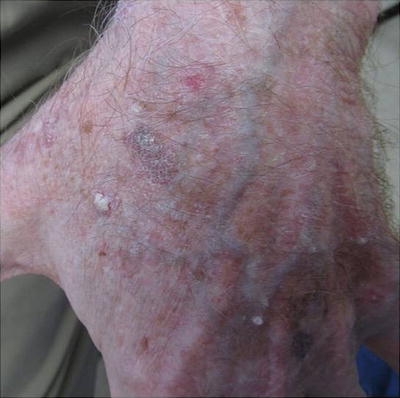
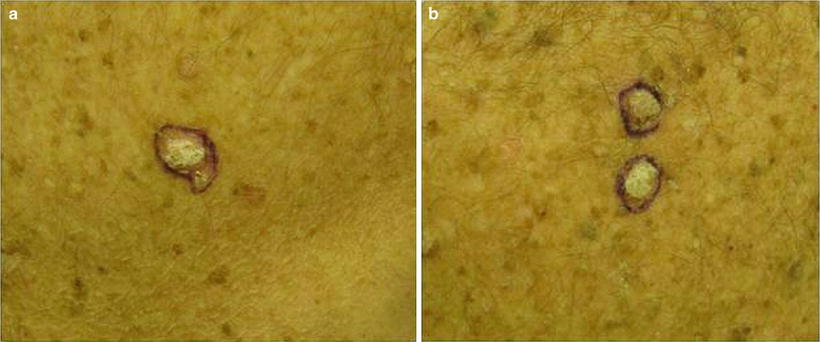

Fig. 18.3

Fig. 18.4
Difficulty distinguishing verruca vulgaris (VV) from squamous cell carcinoma (SCC). Both images show similar appearing pink keratotic papules on the back of a renal transplant patient. Skin biopsy of (a) revealed SCC. Pathology of two lesions in (b) demonstrated verrucae. Since it can be challenging to distinguish VV (nonmalignant lesion) from SCC (malignant lesion) on clinical presentation alone, it is important to have a low threshold to perform skin biopsy
Squamous Cell Carcinoma In Situ (SCCIS)
SCCIS present as pink hyperkeratotic papules or plaques, most commonly on sun-exposed areas (see Fig. 18.5). It can be difficult to distinguish between SCCISs and AKs since both are found on sun-exposed areas; SCCIS tends to be thicker and larger with a well-demarcated border [68]. The terms SCCIS and Bowen disease are sometimes used interchangeably. Bowen disease connotes a viral (HPV) etiology. In non-sun-exposed areas, the etiology of SCCIS is invariably HPV-related.
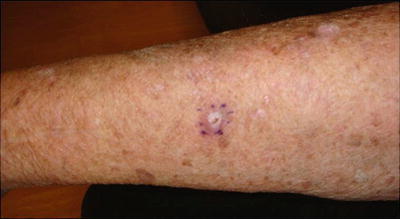

Fig. 18.5
Squamous cell carcinoma in situ (SCCIS). SCCIS presents as pink hyperkeratotic papules or plaques, most commonly on sun-exposed areas. Compared to AKs, SCCIS tends to be thicker and larger with a well-demarcated border. In the non-sun-exposed areas, SCCIS is almost always related to HPV infection
Field Cancerization
Some patients with severe photodamage, especially those on long-term immunosuppression, can develop malignant and premalignant lesions that are so numerous that they become confluent and cover large body surface areas such as the dorsal hands and forearms (see Fig. 18.6). This phenomenon, referred to as “field cancerization,” describes “dysplastic fields of skin” with varying amounts of genetic abnormalities triggered by UV exposure. These are high-risk areas in which premalignant and malignant lesions frequently develop [69, 70].
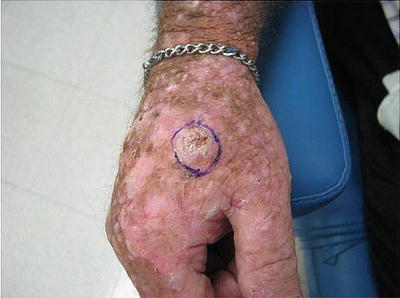

Fig. 18.6
Squamous cell carcinoma developing within an area of field cancerization. Hyperkeratotic pink nodule developing within a field of pink scaly papules and photodamage on the dorsal hand. Field cancerization refers to the development of “dysplastic fields of skin” with differing amounts of genetic abnormalities due to UV damage. Premalignant and malignant lesions can arise within these diffusely photodamaged areas. Vigilant sun protection and cyclical use of field therapies (including topical creams and photodynamic therapy) are essential to the management of field cancerization
Squamous Cell Carcinoma (SCC)
SCCs can vary in presentation but are typically firm, flesh-colored or pink, keratotic papules or plaques or nodules (see Fig. 18.7a–c). They may be smooth or ulcerated. Alternatively, a cutaneous horn may be prominent.
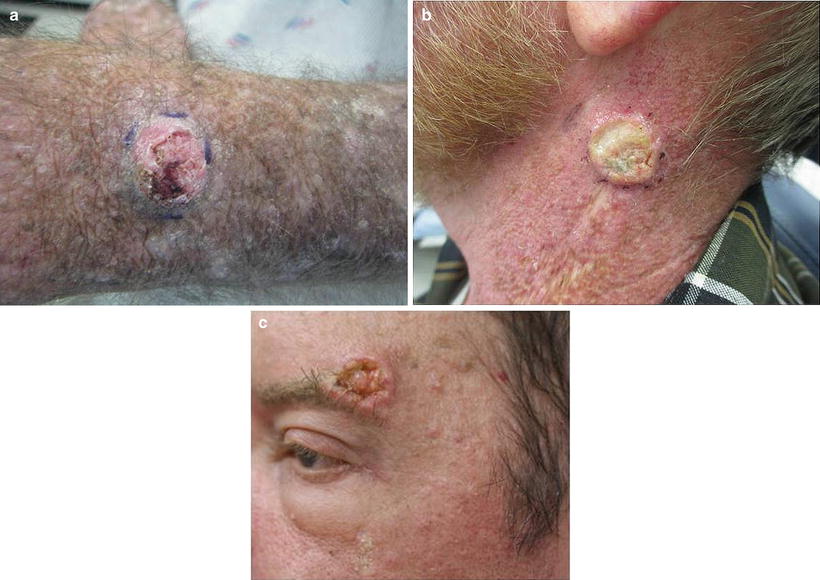

Fig. 18.7
Squamous cell carcinoma (SCC). Renal transplant recipients (RTRs) are at 65 times higher risk of developing SCCs compared to the general population. SCCs are a significant cause of morbidity and associated with a 5–8 % risk of mortality in RTRs. Etiologies include type and duration of immunosuppression, past sun exposure and skin type, and HPV status. (a) Typical SCC developing in a sun-exposed area: eroded hyperkeratotic pink dome-shaped nodule on the dorsal hand. (b) SCC on the neck: Rapidly growing hyperkeratotic nodule. In this anatomic location, there is a high suspicion for lymph node involvement. (c) SCC developing during rapamycin therapy. Though rapamycin-based regimens are the immunosuppression regimen associated with the lowest incidence of SCC, patients may still develop SCCs while on rapamycin therapy
SCCs occur primarily on sun-exposed areas but may be seen in non-sun-exposed areas particularly if HPV-induced. It has been reported that SCCs in immunosuppressed patients have higher rates of both HPV infection overall and with high-risk HPV subtypes compared to the general population [71]. Further studies on the pathogenic role of HPV in skin cancer may have important therapeutic implications in RTRs since prophylactic vaccination could theoretically help to prevent skin cancer development. For now, identifying SCCs at an early stage is particularly important in RTRs as they have a propensity to metastasize and have worse outcome [72–74].
Rapid lesional growth should provoke concern for aggressive behavior and the potential for metastatic spread. SCCs that develop on the ear, lip, scalp, and dorsal hands are at a higher risk for metastatic disease in SOTRs [75–77]. SCCs can be divided into low-risk or high-risk tumors based on overall risk for metastatic spread or local recurrence; risk ultimately determines the definitive treatment. High-risk tumors prompt aggressive surgical management. For low-risk tumors, less aggressive destructive or nonsurgical methods may be indicated.
High-risk features for SCCs in SOTRs are listed in Table 18.2.
Table 18.2
SCC high-risk features in SOTRs
Anatomic location | Ear, lip, scalp, dorsal hands, eyelid, nose (any size) |
Size | >2 cm in any anatomic location >1 cm in intermediate risk area (cheeks, forehead, neck) >0.6 cm in high-risk area (“mask areas of face,” genitalia) |
Histologic features | Poorly or moderately differentiated subtype Acantholytic subtype Perineural invasion |
Clinical characteristics | Recurrent |
Basal Cell Carcinoma (BCC)
BCCs appear as pearly pink papules with telangiectasias (that may resemble a pimple) on sun-exposed areas (see Fig. 18.8). While BCC rates are increased tenfold in SOTRs compared to the immunocompetent population, they are much less frequent than SCCs. Immunosuppression per se is believed to be responsible for the increased number of BCCs; however, the total level of immune suppression is not believed to be relevant to the development of BCC as it is in the development of SCC. The incidence of BCCs in liver/kidney/heart SOTRs was fairly equal, in contrast to the incidence of SCCs previously discussed [16].
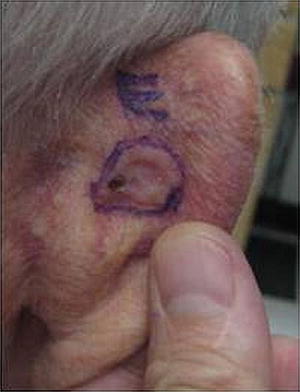

Fig. 18.8
Basal cell carcinoma (BCC). Example of a classic presentation of BCC: pearly ulcerated plaque with rolled border on the posterior ear. BCCs are the most common skin cancer in immunocompetent patients, with 4 BCCs diagnosed for every 1 SCC. In renal transplant recipient (RTRs), the ratio is switched and SCCs are much more common than BCCs (4:1)
Melanoma
Of the four subtypes of melanoma (superficial spreading type (SST), nodular, lentigo maligna, and acral lentiginous), SST melanoma is most common. The characteristic SST melanoma possesses most or all of the following (ABCDE) characteristics for melanoma: asymmetry (A), irregular borders (B), irregular pigment/color (brown, black, whitish, red, and/or blue hues) (C), large diameter (D), central elevation or the lesion is changing or evolving (E) (see Fig. 18.9a, b). While most lesions are pigmented, depigmented, amelanotic (pink or flesh-colored) melanomas (5 %) can be seen [78].
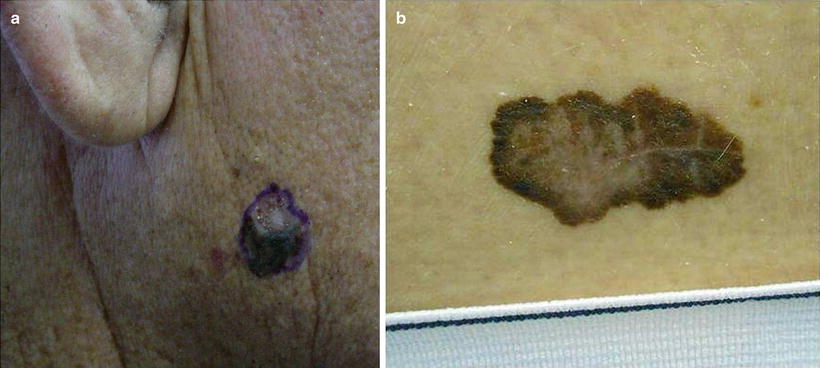

Fig. 18.9
Melanoma. Melanomas in renal transplant recipient (RTRs) have a similar appearance to those in the immunocompetent population with slightly lower survival rates, but overall similar disease course. (a) Irregularly colored (dark brown and pink) irregularly bordered nodule on the cheek. (b) Large irregularly pigmented (brown, black, and pink) patch with a scalloped border on the back
Melanoma is more common in patients with lighter skin types in both immunocompetent and immunocompromised patients. While the prevalence is overall low in RTRs, it is striking that the rate of melanoma in African-American RTR patients is 17.2 times that of the general African-American population [79].
Kaposi Sarcoma (KS)
KS is caused by reactivation of/or recent inoculation with human herpesvirus 8 (HHV-8) and commonly presents as a red-to-purple or dark blue papular or nodular vascular tumor on the legs and arms (see Fig. 18.10). KS is often a systemic disease affecting the gastrointestinal (GI) tract, lymph nodes, liver, and lungs [80, 81].
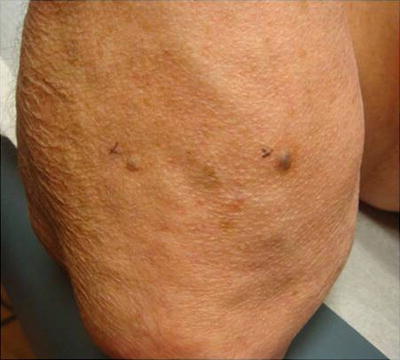

Fig. 18.10
Kaposi sarcoma. Characteristic presentation of Kaposi sarcoma: Dark blue papular vascular tumor near the elbow. Kaposi sarcoma is almost exclusively seen in immunosuppressed patients and is caused by reactivation or new human herpesvirus 8 (HHV-8) infection
Merkel Cell Carcinoma (MCC)
Though still rare overall, rates of MCC are 5–10 times higher in immunosuppressed patients than in the general population [82]. MCC usually presents as a firm, painless, rapidly growing, flesh-colored, red or blue nodule, on sun-exposed areas (see Fig. 18.11).
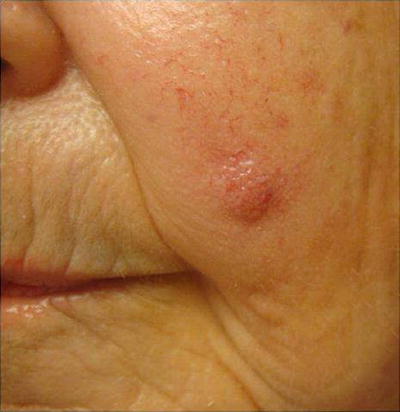

Fig. 18.11
Merkel cell carcinoma (MCC). A common presentation of MCC: rapidly growing asymptomatic nondescript pink nodule on a sun-exposed area. Merkel cell carcinoma pathogenesis has been associated with Merkel cell polyomavirus (MCV) infection. MCC tends to develops at an earlier age in immunosuppressed patients than the immunocompetent population, with lower survival rates. Three-year MCC-specific survival rates are much lower in patients with history of immunosuppression compared to those without (40 % vs. 74 %) [85]
Approximately 80 % are caused by the Merkel cell polyomavirus (MCV); however other contributing factors must be present for malignant transformation since MCV seropositivity is common in the healthy unaffected general population [83].
Management
Preventative Measures
All patients who undergo kidney transplantation will benefit from adhering to sun protective behaviors. Unfortunately, although most SOTR patients are aware of their increased risk of skin cancer, most RTRs do not prioritize reducing their risk among their health concerns [86, 87].
Because immunosuppression is greatest during the acute transplant phase and skin cancer risk correlates with intensity of immunosuppression, it is important for patients to begin avid sun protection immediately after transplantation and to maintain sun protective behaviors throughout their lives. Skin cancer risk appears to be cumulative and continues to increase over time from transplantation [88]. In Queensland, Australia, Ramsay (2002) et al. reported that the cumulative incidence of skin cancer in RTRs after 20 years was 82 % [89].
The benefits to sun protection are not theoretical; regular sunscreen use has been shown to decrease AK and invasive SCC (and, less so BCC) development in SOTRs [87]. Education is key and repetition is necessary to increase compliance. A list of sun protective recommendations is seen in Table 18.3.
Table 18.3
Sun protective behavior recommendations
•No indoor tanning |
•Avoid prolonged time outside or frequent outdoor activities |
•If going outside, avoid peak sunlight hours—10 a.m. until 2 p.m.—when the sun’s rays are the strongest |
•Sun protective behaviors should become routine to patient and employed daily |
–Even when cloudy (as ~70 % of sunrays may still infiltrate) and in the winter |
•Apply a “broad-spectrum” sunscreen, one that protects against both UVA and UVB rays with an SPF 30 or higher |
–Lotions provide the best coverage, but patients should utilize their preferred formulation (e.g., spray, cream, waxy stick) to increase compliance |
–SPF only refers to product’s ability to block UVB rays |
–Products with zinc oxide or titanium oxide ingredients or “helioplex” or “anthelios” on the label provide UVA protection |
–Ideally, two coats of sunscreen should be applied 15–30 min before going outside to provide optimal coverage |
–Reapply sunscreen every 1–2 h, especially after swimming of heavy perspiration |
•Wear wide-brimmed hats, sunglasses, and clothes with sun protective factors (UPF >50) built into the fabric |
Under no circumstances should SOTRs continue indoor tanning. Patients may engage in normal outdoor activities, but prolonged time outside is ill advised. If outdoor activities (e.g., golf, running, or tennis) are important to patient’s quality of life, we recommend that patients avoid going outside from 10 a.m. to 2 p.m. when the sun is the strongest. Wide-brimmed hats or clothes with ultraviolet or sun protective factor (UPF or SPF) >50 built into the fabric are also advised. Sun protective behaviors, especially sunscreen use, should become part of the patient’s daily routine and employed even when cloudy (as ~70 % of sunrays may still infiltrate) and in the winter.
As noted above, daily sunscreen use is the cornerstone to effective photoprotection. Lotions provide the best coverage, but patients should utilize their preferred formulation (e.g., spray, gels, cream, wax stick) to increase compliance. The term “broad spectrum” should be on the product label which indicates both ultraviolet A and B (UVA/UVB) protection.
Products with SPF >30 are recommended. SPF refers to the compound’s ability to block UVB-induced damage only (not UVA). The SPF calculation is based on the application of one teaspoon to the face or one ounce (golf ball size amount) of lotion to the entire body; application of less decreases the actual SPF in an exponential fashion. A recent study found that most patients typically apply 25–50 % of the recommended amount, which results in an actual SPF of ≤33.3 % of the labeled SPF [90]. Consequently, even a patient who is trying to practice good sun protective behaviors by applying SPF 30 lotion may be inadequately protected if minimal amounts are used, resulting in an effective SPF equivalent to ten or less. Recent studies show that use of higher SPF sunscreen (SPF 70 or SPF 100) may be one way to offset the inadequate amount of sunscreen typically applied [91]. If a patient were to apply 25–50 % of the recommended amount of an SPF 100 sunscreen, in theory the real-life SPF would be approximately 30, the recommended SPF. In 2011, the Food and Drug Administration (FDA) issued more stringent guidelines for the definition of SPF and other terms on sunscreen labels. For now, the FDA classifies sunscreens with SPF 70 or 100 together in one group “SPF 50 or higher” and has yet to decide if SPF 70 or SPF 100 products have any definitive added protective benefit [92].
Terms such as “helioplex” or “anthelios” or the ingredients zinc oxide or titanium oxide denote products with UVA coverage. “Helioplex” and “Anthelios” are patented compounds made by Neutrogena and La Roche-Posay, respectively [93, 94]. Aveeno makes a similar compound called “active photobarrier complex” [95]. All of these products contain oxybenzone and avobenzone as well as other chemicals and are formulated to provide both UVA and UVB coverage and to resist photo-degradation. The same ingredients are utilized in most sunscreens and one need not spend a lot of money to ensure adequate protection. As a matter of fact, a recent Consumer Reports analysis found that cheaper generic store brands actually provided better protection than the pricier brands [96].
Unlike oxybenzone or avobenzone, which are organic compounds and UV absorbers, titanium oxide and zinc oxide are physical blockers, which primarily work by reflecting and scattering sunlight particles. Titanium oxide and zinc oxide may bring to mind images of lifeguards with white noses or ghost-like faces, which may make patients hesitant to use them. This should no longer be a concern as the new formulations of titanium and zinc oxide are made of smaller nanoparticles, which blend in and are not as noticeable [92].
There are no “waterproof” sunscreens and almost all products will be inactive within 2 h. In fact, the phrase “waterproof” and other similar terms such as “sunblock,” “waterproof,” “sweat resistant,” and “all day protection” are now prohibited from sunscreen labels as they were deemed inaccurate and misleading by the FDA. In its place, sunscreen labels may have the statements “water resistant (40 min)” and “very water resistant (80 min)” on them. These are terms derived from studies where the SPF was measured before and after subjects were immersed in a whirlpool for 40 min (twice for 20 min) or 80 min (four times for 20 min) [92].
Ideally, two coats of sunscreen should be applied 15–30 min before going outside to provide optimal coverage. Frequent reapplication, every 1–2 h, is necessary for extended periods of time outside.
Many ingredients in sunscreens (in particular oxybenzone) are known to cause allergic contact dermatitis, a delayed-type hypersensitivity reaction in some individuals [97]. If a contact dermatitis is noted after wearing sunscreen, this should not deter the patient from continuing good sun protective behaviors. While patients should discontinue using the offending product, they can continue wearing sunscreen with physical blocker ingredients only (titanium oxide or zinc oxide), which have very minimal to no allergenic potential. Other recommended options include wearing UPF clothing and a wide-brimmed hat as noted above.
It is important to monitor vitamin D levels in SOTRs and supplementation; oral supplementation should be used as necessary. For SOTRs there is a consensus that adequate photoprotection to decrease the risk of skin cancer outweighs the risk of low vitamin D levels since vitamin D can be supplemented [98, 99].
Frequency of Skin Exams
Detection and treatment of existing skin cancers and precancers PRIOR to transplantation is optimal. After transplantation, the frequency of regular skin exams is based on disease burden as depicted in Table 18.2. It is recommended that an FBSE be performed at least every 6 months in patients at risk. Those who develop multiple SCCs may require closer monitoring ever 1–3 months [76].
Management of Actinic Keratoses
Aggressive treatment of AKs or warts with cryotherapy is recommended if there are only a few lesions. For more extensive field damage or numerous AKs, cyclic field therapy is recommended.
Cryotherapy
Cryotherapy involves the application of liquid nitrogen (LN2) onto the skin; duration is determined by anatomic site and lesion type being treated. The LN2 causes rapid cellular freezing, which leads to tissue necrosis. It can be highly efficacious treatment for most AKs and warts. However, for thicker or hyperkeratotic AKs or more diffuse actinic damage, it is not adequate [70].
Field Therapy
Field therapies are employed to treat large photodamaged areas and/or diffuse AKs [70]. Field therapies will not penetrate deep enough to adequately treat skin cancers; one benefit of field therapy is allowing for the visualization of discrete cancerous lesions once the actinic background improves. This may be mistaken for lack of effectiveness but should not dissuade clinicians from continuing field therapy.
Diclofenac
Diclofenac sodium 3 % gel is a topical nonsteroidal anti-inflammatory drug (NSAID) that works via cyclooxygenase 1 and 2 (COX1, COX2) inhibition. It halts AK development by blocking the formation of angiogenic and tumorigenic factors produced from downstream arachidonic acid metabolites.
The recommended regimen for topical 3 % diclofenac is twice daily application for 60–90 days [100, 101]. Relatively low response rates (40 %) are balanced out by increased tolerability compared to other topical therapies. Side effects include mild pruritus, erythema, dry skin, hyper- and paresthesia, and type IV hypersensitivity reactions. Systemic drug interactions similar to that seen with oral NSAIDs are not expected with topical diclofenac therapy. Studies from the rheumatologic literature report that the maximal plasma concentrations following topical application of diclofenac and NSAIDs to body surface areas greater than 100 cm2, but less than 200–300 cm2, were less than 15 % of the plasma concentration reported when comparable doses were administered orally [102, 103]. In three clinical trials testing 3 % diclofenac gel to treat AK lesions, serum levels drawn after 105 days of twice daily application to body surface area of up to 75 cm2 (slightly smaller than area of one’s face and typical area of treatment in clinical practice) were extremely low, on average ≤20 ng/mL [104]. By comparison, the area under the curve (a measure of systemic absorption used in pharmacokinetic studies) of a single oral 75 mg dose of diclofenac (Voltaren®) is 1600 ng/h/mL [104]. Topical diclofenac also does not appear to affect renal function. Creatinine elevations were observed in a small fraction of patients (2 of 48) who were treated topical 3 % diclofenac during clinical trials, but this was the same rate as observed in the placebo group (2 of 49) [104].
Topical 5-Fluorouracil
Topical 5-fluorouracil (5-FU) inhibits thymidylate synthetase and subsequently halts DNA synthesis in cutaneous carcinogenesis. Its therapeutic effects were discovered as a fortuitous side effect; patients receiving intravenous 5-FU as a systemic chemotherapy were noted to have inflammation and resolution of AKs. Twice daily application of topical 5-FU for 2–4 weeks is indicated for the treatment of AKs; a longer course consisting of at least 3–6 weeks is needed for superficial BCCs. Response rates of 90 % have been reported in immunocompetent populations [23, 105]. While response rates are higher than observed with diclofenac, adverse effects (AEs) from 5-FU often lead to early discontinuation; thus, the true response rates are difficult to establish. Significant crusting, redness, erosions, and sometimes pain can be seen during topical 5-FU treatment. Eroded skin may become superinfected, with a great concern for infection with methicillin-resistant Staphylococcus aureus (MRSA). Pharmacokinetic studies have shown that systemic absorption of 5-FU is minimal (2.4 %) [106].
Topical 5-FU can also be used as part of a “chemowrap,” which is a compression bandage that is applied in the office after topical application of 5-FU allowing for prolonged and adequate 5-FU penetration. Chemowraps are commonly employed to treat large areas of skin where it is difficult to apply 5-FU twice daily and/or extensive, severely photodamaged areas amenable to wraps, like the legs. After covering the leg with 5-FU, medicated petrolatum and zinc oxide impregnated gauze are applied. The top layer consists of Coban™, a self-adherent elastic bandage akin to a disposable ACE bandage. “Chemowraps” are replaced every week in the office. Reduction of malignant or premalignant lesions seen 6 weeks following a chemowrap treatment regimen of 4–20 weeks may be maintained for 3 years of follow-up [107, 108]. Chemowraps may also be useful in patients who cannot tolerate surgery.
Imiquimod
Imiquimod is a topical cream that is effective for the treatment of AKs, warts, and superficial BCCs in the RTR as well as in the immune competent patient [109, 110]. Imiquimod is a toll-like receptor (TLR) 7 agonist, which triggers a local immune response through upregulation of numerous cytokines including interferon (IFN)-alpha and IFN-gamma [111, 112]. Though it is generally considered safe, its use in SOTRs was initially limited due to concerns that the immunomodulation it would induce might increase systemic IFN levels causing an increase in graft rejection [113]. However, a large randomized trial demonstrated no increase in graft rejection [112]. The defined treatment area for these studies was 100 m2, essentially the entire area of a face which is the largest surface area that would be treated in clinical practice at one time [112]. Although imiquimod may be used for field therapy, it is more often used to treat discrete precancerous and cancerous lesions, which limits the possibility of systemic absorption. Efficacy (based on 3–5 times per week application for 16 weeks) is reported to be similar in SOTRs and in nontransplanted populations (~70 %) [112]. Side effects are similar to that of 5-FU (erythema, inflammation, itching, pain) but tend to be milder. Some patients may exhibit exuberant inflammatory response, which may be indicative of good therapeutic effect. Systemic AEs, simulating a flu-like illness, have also been described with imiquimod.
Ingenol Mebutate
Ingenol mebutate is a newer topical medication recently FDA-approved for AK treatment in immunocompetent patients. It is a biologically active compound extracted from a milkweed plant, Euphorbia peplus. No studies exist on its effectiveness in SOTRs. Unlike other topical field therapies, ingenol mebutate only requires 2–3 days of treatment as compared to several weeks [114]. While current evidence suggests that it may be less efficacious than other topical field therapies, it can provide durable results in a subset of patients who demonstrate a good initial response [115]. Long-term lesion reduction and sustained lesion clearance (~87 %) at 12 months have been reported in this same subset of superior responders.
Photodynamic Therapy (PDT)
PDT differs from other field cancerization options as it is an in-office procedure. Upon arrival, patients are pretreated with a topical photosensitizer such as aminolevulinic acid (ALA) or its methyl ester methyl aminolevulinate (MAL). After 1–2 h of incubation patients are exposed to a device with either red light (635 nm) or blue light (410 nm) for ~15 min. The combination of oxygen, the absorbed photosensitizer, and the chemical-specific light wavelength causes cell death in rapidly dividing cells via production of reactive oxygen species (ROS) [70, 113, 116]. While more extensively studied as a treatment for AKs, PDT has similarly excellent rates of clearing BCCs, particularly superficial BCC. Complete clearance rates for superficial BCCs are as high 85–93 % at 3 months [117]. Like other topical field cancerization treatments, depth of penetration is a limiting factor in its effectiveness. PDT is most effective on areas with thinner skin such as the face and neck. In areas with thicker skin, such as the hands or arms, PDT is less effective.
As a chemopreventive modality, studies have shown clearance of AKs for up to 3 months in SOTRs after 2 sequential PDT sessions performed 1–2 weeks apart [113, 118]. Cyclical ALA-PDT (every 4–8 weeks) has also been demonstrated to decrease the incidence of new SCCs by 95 % after 2 years of treatment [119, 120]. Side effects include discomfort and burning during treatment and posttreatment swelling (especially periorbitally), infections, and redness; in addition, herpes simplex virus (HSV) reactivation has been reported [121]. Strict sun avoidance for 48 h after each treatment is required because of prolonged photosensitivity. Since PDT is an in-office procedure, patient compliance is not an issue. However, the time commitment (frequent 2–3 h-long visits over 2 years) proposed for SOTR regimens may be difficult to incorporate into a patient’s schedule.
During field therapy treatment, it is important to biopsy any “ugly duckling” lesion or any lesion that fails to respond to therapy. While field cancerization is useful for most SOTRs, it is not recommended for SOTRs in whom effective chemoprevention (see below) is necessary.
Systemic Chemoprevention
Systemic chemoprevention may be appropriate in a select population of SOTRs: those with multiple [5–10] SCCs per year or multiple SCCs in high-risk locations, or SOTR with a history of leukemia/lymphoma [76].
Retinoids
Retinoids are vitamin A derivatives that have been used in the treatment and prevention of actinic keratoses and NMSCs. They may also be effective adjuvant therapy following surgery for SCC or in patients who cannot tolerate surgery.
Retinoids may work by altering the keratinocytes’ cell cycle and reducing the number of malignant/premalignant lesions while on therapy. Unfortunately, once therapy is discontinued, rebound development of eruptive AKs and SCCs can be observed. Side effects are common and include mucocutaneous dryness, scaling/desquamation, itching, increased cholesterol and triglyceride levels, transaminitis, joint pains, hair loss; side effects may limit dose and duration of therapy. Laboratory monitoring with liver function tests (LFTs) and lipid profiles is recommended every 3 months. Of note, all retinoids are teratogenic; female patients must be counseled to avoid pregnancy during treatment. Acitretin should not be used in women of childbearing potential since its teratogenic effects last 3 years after discontinuation.
Oral Capecitabine
Capecitabine, an oral prodrug of 5-FU, has recently been studied for NMSC chemoprophylaxis in SOTRs. It is currently FDA-approved for use in metastatic breast and colon cancers [76, 127]. Capecitabine is metabolized by dihydropyrimidine dehydrogenase (DPD) and pretreatment DPD serum levels should be checked as DPD deficiency may lead to significant toxicity. The efficacy for capecitabine as a chemopreventive measure is based on a relatively small cohort of SOTR patients, with only 15 study patients in the largest report, of whom 80 % were RTRs. Success depended upon lesion type; incidence rates dropped most markedly for AKs (2.45×), with modest decreases in rates of SCCs (0.33) and BCCs (0.04) seen at 12 months [128]. Severe toxicities included fatigue (40 %), hand-foot syndrome (20 %), and diarrhea (20 %) [128].
Treatment of Skin Cancers
Outright NMSCs should be managed by surgical means whenever possible and proceed in concert with ongoing treatment of precancers/field cancerization. The focus of this section is the treatment of NMSCs, primarily SCCs. Management of melanoma, KS, and MCC is discussed briefly.
Melanoma
All melanomas should be treated with wide local excision (WLE) based upon the NCCN or AAD guidelines. The margin of excision and indication for sentinel lymph node biopsy (SLNB) is primarily dependent on the Breslow depth (BD) of tumor; margins recommended for lesional thickness are for in situ: 0.5 cm; for lesions less than or equal to 1 mm BD: 1 cm; for lesions between 1.0 and 2.0 mm BD: 1.0–2.0 cm: for lesions over ≥2.0 mm; 2.0 cm. Survival rates of SOTRs with melanoma are lower than those in nontransplanted patients with similar BD; thus, more aggressive management may be considered for lower risk melanomas in the SOTRs [129].
Kaposi Sarcoma (KS)
Studies report KS lesions limited to the skin have been treated with imiquimod, lasers, surgery, cryotherapy, or radiotherapy [80]. However, radiation for KS has been associated with an increased risk of NMSC [81].
For more extensive cases of KS, decreasing immunosuppressive therapy or switching to rapamycin-based regimen can improve the outcome in RTRs. It is thought that lower immunosuppression is responsible for KS regression, not the antiproliferative effects of sirolimus, as KS recurrence has been reported in sirolimus-based regimens when patients experienced high sirolimus levels [130, 131]. Advanced or systemic KS, unresponsive to the withdrawal of immunosuppression, has been treated somewhat successfully with chemotherapy, particularly with taxols [80




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








