Minimizing and Treating Complications
 INTRODUCTION
INTRODUCTION
The most commonly encountered complications of treatments for venous insufficiency are telangiectatic matting (TM), hyper-pigmentation, and cutaneous necrosis. The first two of these problems are minor from a medical standpoint, and hopefully any necrosis would also be minor, but all three can be very distressing to the patient. Fortunately, meticulous technique can reduce the incidence and severity of these problems to a very low level. Two additional complications that are not minor are inadvertent arterial injection and inadvertent nerve injury. Prevention of these serious problems is critical, as there is no treatment that can prevent a bad outcome once these injuries have occurred.
 CUTANEOUS NECROSIS
CUTANEOUS NECROSIS
Etiology
There are several causes for cutaneous necrosis. The most common cause is the extravasation of sclerosant into perivascular tissues. This extravasation necrosis is most common with hypertonic saline (HS), is seen with some regularity with most other agents, but is rare with polidocanol (POL) and glycerin. Extravasation necrosis is rare in experienced hands but is relatively common when a practitioner with little experience performs treatment.
A second type of cutaneous necrosis does not come from extravasation, but rather from back-flow of sclerosant through an unsuspected cutaneous arteriovenous malformation, to produce sclerosis of an end-arteriole with subsequent necrosis of the cutaneous tissues depending on that arteriole. This type of necrosis can occur with the injection of any sclerosing agent even under ideal circumstances and does not necessarily represent physician error. Arteriolar spasm is believed to play a role in many of these cases.
Cutaneous necrosis may also result from excessive local compression or from excessive traction on the skin (usually due to tape). Another cause for necrosis, direct intra-arterial injection, should be avoidable by a proper technique. One should cease injection immediately if a patient complains of sudden severe pain, as arterial puncture is much more painful than venipuncture.
EXTRAVASATION During injection of an abnormal vein or telangiectasia, even the most adept physician may inadvertently inject a small quantity of sclerosing solution into the perivascular tissue. In some cases, sclerosant may flow back out of the vein when the needle is withdrawn. In others, vascular spasm causes the needle tip to pass completely through the small vessel, allowing sclerosant to be deposited in the deep perivenous tissues. Fragile veins may tear during needle placement, allowing immediate leakage during injection. A patient may unexpectedly move during the injection, causing the needle to shift out of the vessel.
Sclerosing solutions vary in the degree of cellular necrosis they produce. HS at 23.4 percent is a caustic sclerosing agent, and when an inexperienced practitioner uses this agent, small spots of superficial epidermal damage are often seen at points of injection where a small bleb of the solution has escaped from the vein.1
In contrast to HS, POL was originally intended for subcutaneous injection as a local anesthetic agent and is only minimally toxic to subcutaneous tissue. Nonetheless, extravasation of POL in sufficient concentration has occasionally been reported to cause necrosis.2,3 Sodium tetradecyl sulfate (STS) is caustic to skin when injected extravascularly and caution must be exercised with its use in smaller telangiectasias. When injected at concentrations of 0.1 percent, small amounts of extravasation are unlikely to cause any epidermal injury. High incidences of cutaneous breakdown have been reported with 0.5–1 percent for superficial telangiectasias.4 Chromated glycerin (CG) solutions, available in Europe, are extremely weak sclerosants that have been reported to produce cutaneous necrosis only rarely. There are no reported cases of ulceration using compounded glycerin-lidocaine solution.
ARTERIOLAR INJECTION Approximately 4 percent of leg telangiectasia have some communication with a dermal arteriole.5 Inadvertent flow of sclerosant through this communication is believed to be a cause of cutaneous ulcerations unrelated to extravasation. Excisional biopsies were performed in a small series of patients who developed ulceration after meticulous injection of tiny telangiectasias using low concentrations of POL, and in every case, there was a classic wedge-shaped arterial ulceration with an occluded feeding dermal arteriole at its center (Figure 24-1).6
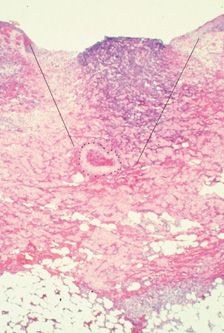
![]() FIGURE 24-1 Histologic examination of 4-mm diameter ulceration after injection with POL 0.5 percent. Note wedge-shaped ulceration with a thrombosed artery at the base (Hematoxylin eosin ×25). (Printed with permission from Mosby).
FIGURE 24-1 Histologic examination of 4-mm diameter ulceration after injection with POL 0.5 percent. Note wedge-shaped ulceration with a thrombosed artery at the base (Hematoxylin eosin ×25). (Printed with permission from Mosby).
VASOSPASM When sclerosant flows into a communicating arteriole, or when sclerosant is extravasated around a dermal arteriole, there is an immediate porcelain white blanching in a roughly circular pattern. The blanching usually persists for many minutes, and in most cases, a hemorrhagic patch forms over the area within 2–48 hr. This patch progresses to a bulla that usually progresses to an ulcer. In many cases, it appears that arteriolar spasm may play some part in this process, as vigorous local massage and the application of a small amount of nitroglycerin ointment 2 percent have been reported to prevent the formation of the bulla and the subsequent ulceration.7
EXCESSIVE COMPRESSION Excessive compression of the skin overlying the treated vein may produce tissue anoxia and lead to localized cutaneous ulceration. Subcutaneous tissue flow in the leg is decreased when cutaneous pressure exceeds 20 mmHg; pressures above 30 mmHg may even reduce blood flow to the muscle in some patients.8,9
Arterial inflow pressure to the legs is greatest when the patient is standing and least when the patient is recumbent. To avoid tissue ischemia, compression should not exceed 30–40 mmHg at night, when the patient will be recumbent for many hours.
If higher levels of compression are needed during periods of ambulation, a double layer of 20–30 mmHg graduated compression stockings should be used. When the patient is recumbent, the outer stocking is removed, thereby decreasing the cutaneous pressure to 20–30 mmHg at the ankle. This level of compression is well tolerated by most patients. Compression is further discussed in Chapter 15.
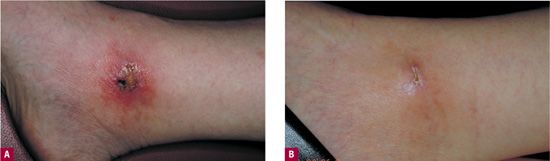
Traction necrosis of the skin occurs when tape exerts a pulling force that interferes with normal skin tension and cutaneous flow. This most often results from improper application of tape but can also develop when patient movement puts excessive traction on the tape that originally was loosely applied (Figure 24-2).
![]() FIGURE 24-2 Friction blister formed from tape, leading to significant superficial ulceration.
FIGURE 24-2 Friction blister formed from tape, leading to significant superficial ulceration.
Prevention
Meticulous technique is the key to prevention of ulceration. Blanching that may indicate cutaneous arteriolar injection or arteriolar spasm is treated with vigorous local massage using a small amount of 2 percent nitroglycerin ointment (to avoid a headache, the clinician should wear gloves when applying nitroglycerin ointment). Careful attention to the application of tape and to the application of a compression garment over tape will help to avoid traction necrosis. Selection of the right size compression garment and the right amount of compression for each patient will prevent compression necrosis.
If a small bleb of low concentration of solution is extravasated, it can usually be massaged for 1 min and will not cause harm to the skin (although this does not always work with HS). It is not known whether extravasated solutions should be diluted by the injection of normal saline. There are case reports of good outcomes when dilution by injection has been performed,1 but prospectively randomized animal studies suggest that efforts to dilute extravasated sclerosants only lead to an increased incidence of ulcers.10 If dilution is attempted after HS extravasation, it is necessary to inject a volume of normal saline at least 10 times that of the extravasated solution to limit osmotic damage. Detergent sclerosing solutions of high strength, such as concentrated STS, are also toxic to subcutaneous tissues if extravasated. Dilution may be attempted, but again, simple dilution has been reported to cause an increase, rather than a decrease in the incidence of ulceration.
Instead of simple dilution with normal saline, dilution with a hyaluronidase solution has been shown to prevent necrosis in a variety of extravasation situations, including an animal model of sclerosant extravasation.10 Hyaluronidase enzymatically breaks down connective tissue hyaluronic acid. It is believed that this may disrupt the normal interstitial fluid barrier to allow rapid diffusion of solution through the tissues, thereby increasing absorption. This beneficial effect reduces extravasation injuries from 10 percent dextrose, calcium and potassium salts, contrast media, sodium bicarbonate, aminophylline, hyperalimentation solution, and doxorubicin.11,12 There are no published studies yet with human data on hyaluronidase and reduction of extravasation damage from sclerotherapy solutions.
In addition to its enhanced dilutional ability, hyaluronidase may independently improve healing and cell survival and reduce scarring in a variety of situations.13 Side effects from hyaluronidase are rare and generally of the urticarial type.14,15
The ideal concentration and quantity of hyaluronidase to inject after extravasation have not been determined. On an empiric basis, 300 USP units of hyaluronidase may be diluted in 5 ml of 0.9 percent sodium chloride and injected into the area of extravasation.
Treatment
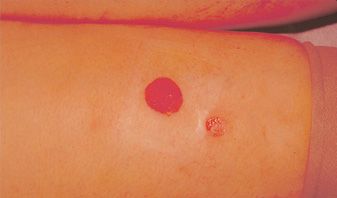
Fortunately, ulcerations, when they do occur, are usually fairly small, averaging 4 mm in diameter. At this size, primary healing usually leaves an acceptable scar (Figure 24-3). Occlusive or hydrocolloid dressings result in an apparent decrease in wound healing time. More importantly, occlusive dressings decrease the pain associated with an open ulcer. Left alone, ulcers may take four to six weeks to completely heal. If this is unacceptable, then excision and primary closure is a reasonable alternative, depending on the anatomic location. Excision of the ulcer with split- or full-thickness grafting usually leads to a worse cosmetic result than healing by secondary intention.
![]() FIGURE 24-3 A. Cutaneous ulceration three weeks after injection of ankle telangiectasia with POL 0.5 percent. An AV malformation was suspected. B. After two months. Complete healing occurred after six weeks of hydrocolloid dressings.
FIGURE 24-3 A. Cutaneous ulceration three weeks after injection of ankle telangiectasia with POL 0.5 percent. An AV malformation was suspected. B. After two months. Complete healing occurred after six weeks of hydrocolloid dressings.
 TELANGIECTATIC MATTING
TELANGIECTATIC MATTING
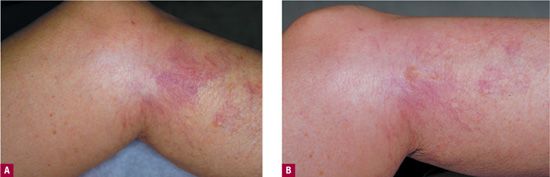
Telangiectatic matting “TM” refers to the appearance of tiny new red (occasionally violaceous) telangiectasias that appear in patients after sclerotherapy or surgical removal of varicose veins or venulectasia. TM presents as “blush type” regions containing large numbers of “new” blood vessels less than 0.2 mm in diameter, located in and around the site of treatment (Figure 24-4). TM is often seen on the thighs, medial ankle, and medial and lateral calves. The inner thigh just above the knee is the most common site of TM.16 This may be due to movement at the knee causing rapid fluctuations in venous pressure or to relative skin anoxia during sleep when the opposite knee in the lateral position compresses this area. Microvessels that remain after treatment of larger telangiectasias can be confused with early TM. Photographs taken before treatment are helpful in distinguishing the two.
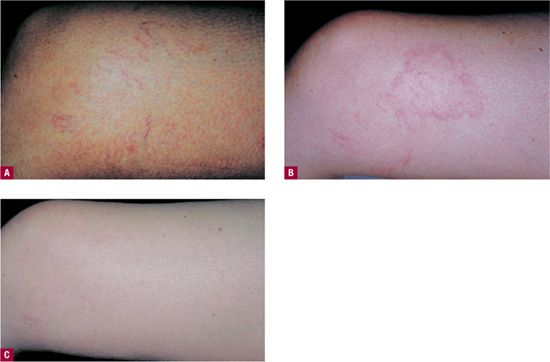
![]() FIGURE 24-4 A. Telangiectasia prior to treatment. B. One month following treatment showing development of TM. C. Spontaneous resolution at six months.
FIGURE 24-4 A. Telangiectasia prior to treatment. B. One month following treatment showing development of TM. C. Spontaneous resolution at six months.
The incidence of TM is between 15 percent and 24 percent.17,18 It is predominantly seen in women, but can also occur in men.16 TM may occasionally be associated with intolerable burning pain and leg edema.19 TM usually resolves spontaneously in 3–12 months.17,20 Occasionally, TM can be permanent and pretreatment patient information should make this clear in an explicit manner.21
Etiology
The precise cause of TM remains unknown, and it is not even certain whether the tiny vessels that make up the blush are dilated vessels that already existed, or whether they are new vessels that have grown into the area.
One theory holds that angiogenic and inflammatory processes cause preexisting subclinical blood vessels to dilate as they carry collateral flow from arteriovenous anastomoses.22 Another theory maintains that matting represents angiogenesis: new blood vessels grow23 as a natural reaction to the sclerotic obstruction of existing vessels and to meet increased metabolic demands caused by perivascular inflammation after sclerotherapy.24,25
Disruption of endothelial continuity and obstruction of outflow from a vessel (the results of sclerotherapy) are physical factors that are known to trigger angiogenesis. Apart from physical factors, more than 40 circulating humoral factors have been implicated in angioneogenesis (Table 24-1). Numerous substances that can inhibit angiogenesis are under active investigation, but none are available for clinical use at this time.26
TABLE 24-1
Biologic Activities of Angiogenic Factors on Endothelial Cell
Risk Factors
Risk factors for TM have been a subject of great debate in the sclerotherapy literature. It is widely believed (though unproven) that TM is more common when higher infusion pressures, larger volumes, and higher concentrations of sclerosant are used. We have observed that when increased infusion pressure is utilized resulting in blanching of the entire capillary network of the skin, TM is more likely to occur. Patient factors associated with an increased risk of TM include excessive body weight, a family history of telangiectasias, and longer duration of spider veins.17 There is a decreased incidence of TM with aging,27 possibly due to a decrease in dermal venular mast cells.28
Exogenous and endogenous increases in estrogen may be an important risk factor for matting,29 but the mechanism for this is not clear. Estrogen is recognized as a receptor-mediated angiogenic factor in a variety of tumors, but conflicting reports of estrogen and progesterone receptors in telangiectasias make the role of hormones in TM uncertain. Most recently, however, estrogen receptor levels were reported higher in varicose segments of varicose veins compared with normal veins.30 In addition, previous studies have shown that human saphenous veins from both sexes express progesterone receptors.31 Yet, when 10 patients with TM were examined for estrogen/progesterone receptors, all were negative. Sadick and Urmacher32 concluded that although estrogen and progesterone may play an indirect role in the development of postsclerotherapy TM via vasodilatory or secondary angiogenic or cytokine release mechanisms, they do not appear to play a primary role in promoting postsclerotherapy neoangiogenesis.
Prevention
Efforts to minimize TM (Table 24-2) are especially important because treatment efforts other than waiting are often not successful. Several principles and techniques have been reported to decrease the incidence of TM.20,33,34 The three most important recommendations are to use the minimal effective concentration of sclerosant for a given vessel, the lowest possible volume of sclerosant to flush the vessel, and the lowest possible infusion pressure to deliver the sclerosant. When a patient who is taking exogenous estrogens demonstrates a tendency toward TM, she may wish to temporarily stop the estrogen during the period of treatment. In the future, TM may be prevented by the addition of antiangiogenic substances to sclerosant solutions.
TABLE 24-2
Minimize Occurrence of Telangiectatic Matting
Use minimal sclerosant concentrations
Limit blanching per injection to an area 1–2 cm in diameter
Use low injection pressures, to avoid backup of solution into capillaries
Encourage weight loss prior to institution of therapy
Consider discontinuing oral contraceptives or hormonal replacement therapy for the duration of treatment
Treatment
The most important first step in treating this complication is to repeat the clinical evaluation of the patient, looking for any previously unrecognized sources of hydrostatic pressure. Transillumination (see Chapter 25 for specific devices) may help to locate a previously unseen reticular vein. At the minimum, Doppler examination of the problem areas should be done to listen for unresolved reflux. Much more information can be gained from a duplex ultrasound examination. The duplex study should be performed in the standing position because previously unrecognized high-pressure reflux from an intermittently incompetent (clinically inapparent) saphenous vein may be the culprit. When the saphenous vein itself is competent, duplex often reveals failed perforators and an unsuspected reticular feeding vessel lying below the subcutaneous tissues. Treatment of this vessel by sclerotherapy or by phlebectomy usually causes resolution of the overlying telangiectatic mats (Figure 24-5).
![]() FIGURE 24-5 A. Matting below medial knee arising from hidden incompetent saphenous tributary, found by duplex ultrasound. B. Treatment of the refluxing vessel (which was located just proximal to the medial knee) by duplex-guided sclerotherapy resulted in significant clearing within four weeks. The matting was also treated with IPL on the same day as the duplex-guided sclerotherapy.
FIGURE 24-5 A. Matting below medial knee arising from hidden incompetent saphenous tributary, found by duplex ultrasound. B. Treatment of the refluxing vessel (which was located just proximal to the medial knee) by duplex-guided sclerotherapy resulted in significant clearing within four weeks. The matting was also treated with IPL on the same day as the duplex-guided sclerotherapy.
The second most important part of the treatment plan is simply watchful waiting. The patient is given a mild anti-inflammatory cream to apply to the area, and photographs are taken of the area at six- to eight-week intervals until resolution occurs. Patients can be reassured that even the worst matting can resolve completely over time, and are much more tolerant of this waiting period when they have been suitably informed before the start of treatment that TM may occur.
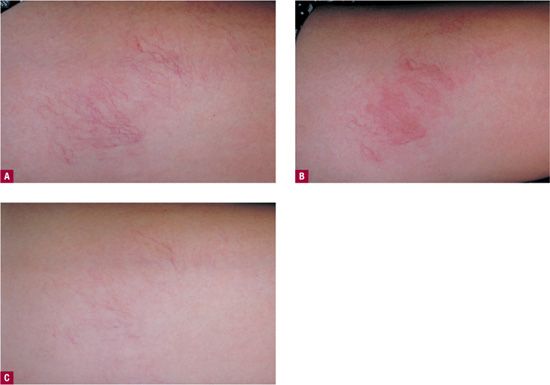
If there is no identifiable feeding vessel and if the matting does not resolve within a reasonable time, then the vessels that make up telangiectatic mats can themselves be treated by sclerotherapy. Disposable insulin syringes and 33-gauge needles can facilitate cannulation of these extremely small vessels. The new double-polariscopic Syris v300 or v600 (Syris Scientific LLC, Shaker RoadGray, ME) may enhance visualization of the entry points into matting, facilitating injection even with a 30-gauge needle. In some patients, the effort is successful, but in other patients, the tiny vessels seem impervious to sclerosing agents. Treatment with high-intensity pulsed light is sometimes effective35 (Figure 24-6) and treatment with pulsed dye laser has also been shown to be somewhat effective in treating matting that has proven resistant to other therapeutic modalities.36 The copper bromide laser may also turn out to be effective but has yet to be tested.37
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree