and Ziv Gil2
(1)
Division of Otolaryngology Head and Neck Surgery and Maxillofacial Surgery, Tel Aviv Sourasky Medical Center, Tel Aviv, Israel
(2)
The Head and Neck Center Department of Otolaryngology Head and Neck Surgery, Rambam Healthcare Campus, Haifa, Israel
Keywords
Free flapFasciocutaneous flapMusculocutaneous flapFascia lataReconstructionHardwareIrradiation12.1 Introduction
Reconstruction of skull base defects caused by resection of a tumor, osteoradionecrosis, osteomyelitis, or trauma is a challenging undertaking. The reconstructive surgeon’s aim is to correct the defects while minimizing potentially devastating complications such as cerebrospinal fluid (CSF) leak, meningitis, osteomyelitis, extrusion of hardware, and severe facial deformity. Doing so requires the ability to segregate the intracranial compartment from the oronasal cavities. It is also important to obliterate all surgically caused cavities, in order to improve contours and minimize the hollow appearance and seroma formation. Hardware and exposed bone should be covered with well-vascularized flaps to decrease the chances of exposure or osteoradionecrosis following the nearly inevitable radiation treatment. The surgeon should also be able to improve outcomes of facial nerve injury either by performing static facial nerve reconstruction procedures or by interposing a nerve graft between the proximal and distal segments.
Most smaller defects associated with skull base surgery can be obliterated by the use of local pericranial, temporalis muscle, or nasal septal flaps. Grafts, such as those taken from the fascia lata or fat, may also be utilized in a well-vascularized environment and for very small gaps. These options have been described in previous chapters of this book.
This chapter deals with the general considerations pertaining to skull base reconstruction and the workhorse free flaps most often used. The specifics of each area and techniques for dealing with the special defects that arise in each area are described.
12.2 Anatomical Considerations
We usually refer to the skull base defects according to the 1994 classification of Irish et al. (Fig. 12.1):
Region I: tumors arising in the orbits and sinuses, extending into the anterior cranial fossa
Region II: tumors originating in the lateral skull base and pterygopalatine fossae
Region III: tumors originating in and involving the ear, parotid gland, and temporal bone, frequently involving the facial nerve to a varying extent
Of course, more than one area may be involved in the defect created by the disease.
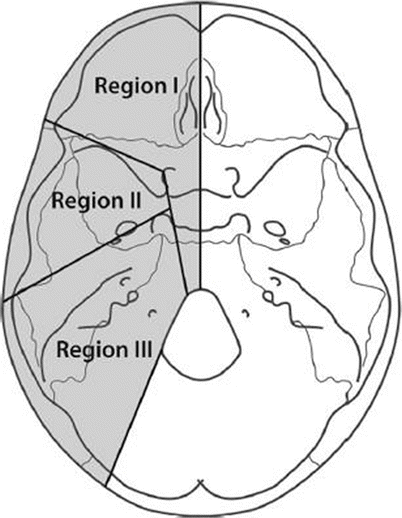

Fig. 12.1
Schematic description of the skull base regions (From Irish et al.; with permission)
12.3 Reconstruction Method
Most patients who undergo free flap reconstruction have already undergone surgery, radiation, and chemotherapy. Younger patients are better candidates for free flap reconstruction because they can tolerate longer and more radical procedures.
12.3.1 Analysis of the Defect and Considerations
The various tissue components needed for reconstruction and their three-dimensional properties must be taken into account when planning a free flap reconstruction. Table 12.1 describes various solutions that are available for the different elements injured during ablative surgery (Figs. 12.2, 12.3, 12.4, 12.5, and 12.6).
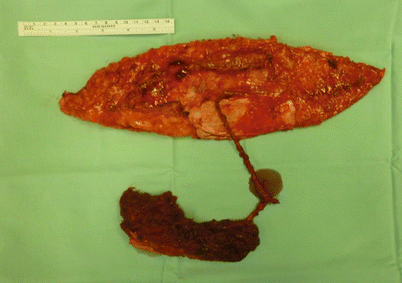
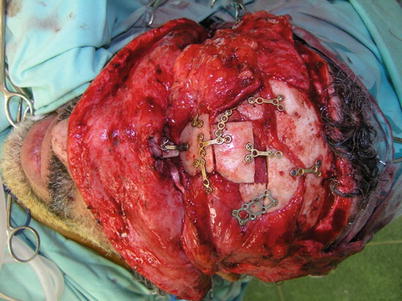
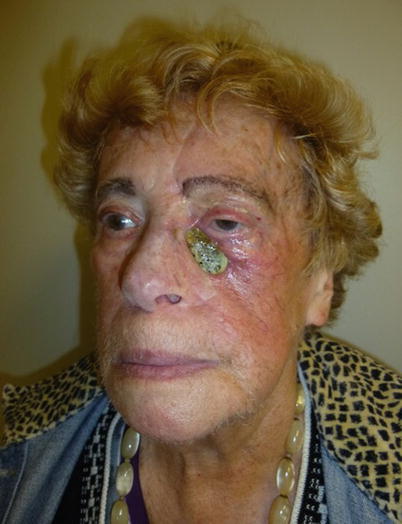
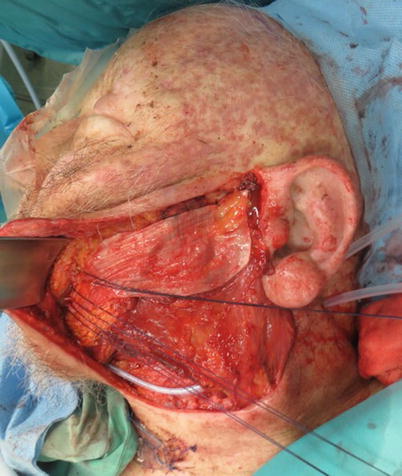
Table 12.1
Surgical defects and their reconstruction options
Expected defect | Reconstruction options |
|---|---|
Violation of dura integrity | Fascia lata as a graft |
Vascularized fascia as partial or whole flap | |
Vascularized muscle | |
Mucosal defect (intraoral, intranasal) | Either a skin paddle facing the cavity or fascia or muscle that is allowed to mucosalize |
Open cavity (maxillary sinus, frontal sinus, mastoid cells) needing obliteration | Inclusion of a “cube” of muscle component as a chimeric flap or as a musculocutaneous flap (Fig. 12.2) |
Contour defect | De-epithelialized skin for maintaining good long-term volume |
Bone graft (Fig. 12.3) | |
Titanium mesh (high extrusion rate after radiotherapy) (Fig. 12.4) | |
Bone flaps (a longer and more demanding procedure, but they serve as a steady scaffold for future dental implants) | |
High probability of radiation to the location | Need to “overfill” muscle components due to atrophy, especially likely after radiation (Fig. 12.5) |
Avoidance of bone grafts, skin grafts, and hardware | |
Planned orbital exenteration, need for an orbital prosthesis | Lining of the orbit with a thin flap (radial forearm) or muscle that will contract to a concavity in which a prosthesis can be fitted |
Location of defect vis-à-vis intended recipient vessels | Choosing a flap with a vascular pedicle measured to size |
Need for ancillary procedures (tissues preferably harvested from the same incision) | Nerve graft for facial nerve restoration taken from the great auricular, sural, lateral femoral cutaneous, or lateral antebrachial cutaneous nerves |
Static sling from the fascia lata, palmaris longus (Fig. 12.6) | |
Dynamic sling from the temporalis muscle | |
Combination of static and functional repair of the nerve for an older patient or one with presurgical weakness or radiation therapy | |
Lower eyelid tightening procedures |

Fig. 12.2
A chimeric flap on two separate perforators: one supplying the muscular component and the other the fasciocutaneous. The muscle component is trimmed to fit and obliterate the sinus cavity

Fig. 12.3
Bony reconstruction using split calvarial bone grafts connected with mini-plates

Fig. 12.4
Titanium mesh can be used for contour, yet following radiation treatment hardware may extrude if not properly covered with well-vascularized tissues

Fig. 12.5
Patient who underwent orbitomaxillectomy, reconstructed with a muscle flap and skin graft over it. Due to radiation, shrinkage of the flap caused distortion of the facial contour and inferior displacement of the lateral eyebrow. These defects should be overcorrected, and the eyebrow should be fixated to the periosteum of the orbital rim

Fig. 12.6
A fascia lata static sling connecting the lateral oral commissure with the zygomatic arch and temporal fascia. The sling overcorrects the position of the commissure, as the fascia relaxes with time and the corner of the mouth will droop
12.3.2 Flap Variations
The optimal choice of flap is decided upon according to the analysis of the defect and its requirements. The following discussion covers the most commonly used flaps.
12.3.2.1 Anterolateral Thigh Flap
The anterolateral thigh (ALT) flap is the most versatile and most frequently used flap in our institution and many others. The ALT flap may be used solely as a fasciocutaneous flap (Fig. 12.7a) if a thin and pliable tissue is required. It may be harvested as a musculocutaneous flap (Fig. 12.7b), with the muscle component tailored to the exact size of the cavity to be obliterated. It can serve as a chimeric flap combined with different tissues (e.g., skin, fascia, and muscle), each on a different vascular branch (Fig. 12.7c). The lateral femoral cutaneous nerve may be harvested for facial nerve cable grafting, or the nerve to the vastus lateralis may be used if that muscle is taken with the flap. Donor-site morbidity is quite minimal, even if the muscle is included in the flap. Patients can be ambulated on day 1 following surgery. If a large skin paddle is taken, the remaining defect can be skin grafted.
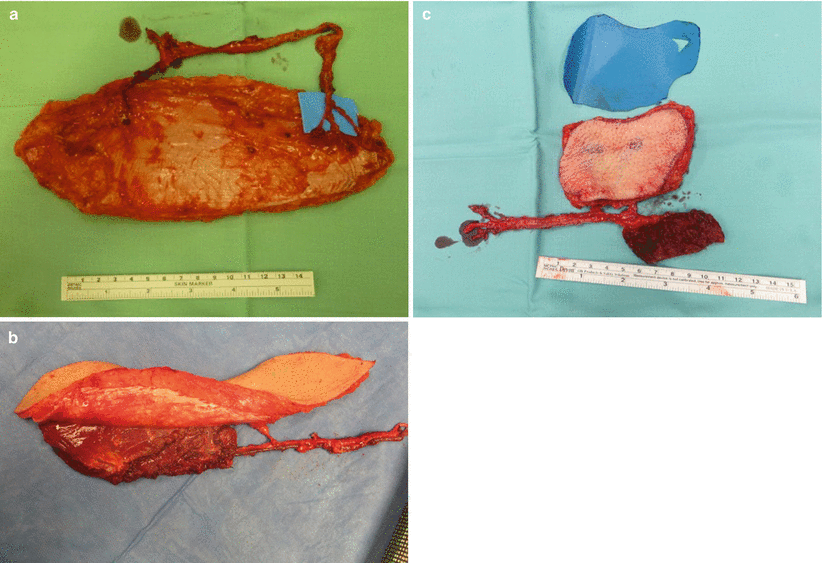

Fig. 12.7
(a) A “pure” fasciocutaneous perforator anterolateral thigh flap, based on two muscle perforators. The perforator on the right splits into three smaller skin vessels. (b) A musculocutaneous flap with the skin and muscle component supplied confluently by the same vessels. (c) A chimeric flap. The muscle component is supplied by a separate side branch as the fasciocutaneous component. This allows for more freedom during inset
The harvesting of an ALT flap involves several steps:
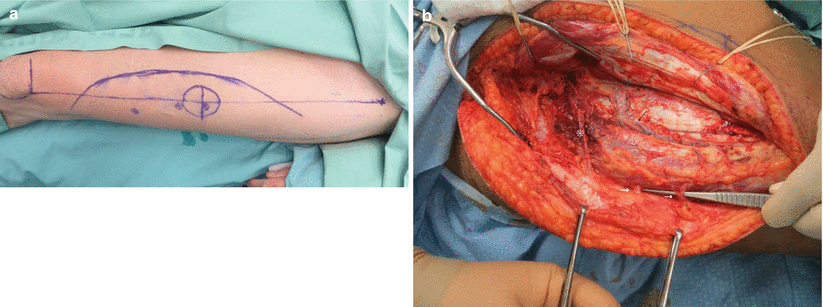
1.
A line is drawn between the anterior superior iliac spine and the lateral border of the patella (the AP line). This line delineates the septum running between the rectus femoris and vastus lateralis muscles. Within this septum lies the major pedicle of the flap, including the descending branch of the lateral circumflex femoral artery and vein (Fig. 12.8a).
2.
A skin perforator can be Dopplered halfway down the AP line and about 1.5 cm lateral to it. Additional perforators may be found with less consistency about 5 cm proximally and distally to the previously described point along the leg’s axis. Perforators may be either septocutaneous or musculocutaneous (Fig. 12.8b). Dissection through the muscle should be performed for a true fasciocutaneous flap (Fig. 12.7a). When needed, a muscle is harvested en bloc with the perforators passing through it (Fig. 12.7b).
3.
The pedicle is dissected proximally, disconnecting all the side branches until it reaches a length and caliber enabling it to anastomose comfortably to the intended recipient vessels (usually at the site where the pedicle contacts the bifurcation of the profunda femoris vessels).
4.
The posterolateral incision of the ALT flap’s skin paddle is incised once the defect can be fully assessed and a template of the skin paddle can be made. (We regularly use a piece of rubber Esmarch bandage to etch the contour of the defect as shown in Fig. 12.7c.)
5.
Recipient vessels are established and a good blood flow through them is verified. A wide tunnel connecting the defect to the vessels is dissected, usually in a subcutaneous plane. Only then can the free flap be disconnected from its original site and transferred to the surgical defect.

Fig. 12.8
(a) The septum between the rectus femoris and the vastus lateralis is delineated by the anterior superior iliac spine (ASIS)–patella (AP) line. Perforators (crosses) are around the mid-distance of this line and approximately a centimeter lateral to it. (b) After the skin and fascia are incised, the septum between the rectus femoris and vastus lateralis is entered. The descending branch of the lateral femoral circumflex artery (black arrow) is seen at the base of the cleft. Here an anatomical variant can be seen. The first cutaneous perforator (asterisk) is septal, yet is based on a separate branch, off the lateral femoral circumflex, namely, the “diagonal branch.” The two more distal perforators (white arrows) branch off the main vascular trunk and are muscle perforators
12.3.2.2 Radial Forearm Free Flap
A radial forearm free flap (RFFF) is also reliable and simple to harvest. It is very thin and pliable and is excellent for resurfacing superficial defects or folding over for internal and external lining (Fig. 12.9). The Allen test is performed prior to its harvest to verify that the radial flow is not the dominant blood supply to the palm and fingers. The flap is harvested from the nondominant arm and is centered over the course of the radial artery, distally limited by the wrist crease. The radial artery and venae comitantes are dissected up to the cubital fossa.
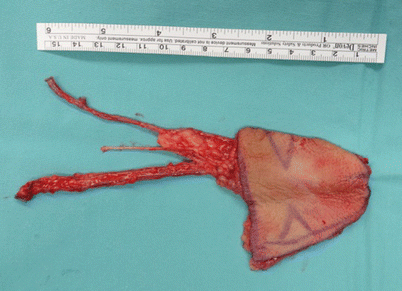

Fig. 12.9
A radial forearm free flap is very thin and pliable. Here the flap is harvested both with the radial artery and the venae comitantes and with the cephalic vein as an additional venous drainage. Additionally, the lateral antebrachial cutaneous nerve is also seen in the middle between the vascular pedicle and cephalic vein and, if connected to a stump of a sensory nerve, may render the flap sensate
12.3.2.3 Rectus Abdominis Myocutaneous Free Flap
The rectus abdominis myocutaneous free flap was the flap of choice to reconstruct maxillary defects, but it has fallen out of favor since the ALT became popular. It can be harvested with one or more skin paddles, including a cube of rectus abdominis muscle, to fill cavities. It is based on the inferior epigastric artery, which allows a fairly long pedicle. Donor-site morbidity is more pronounced, however, as bulges and hernias may result even with mesh reinforcement of the rectus sheath. Additionally, the flap may be too bulky in heavier patients, and a simultaneous two-team approach is more difficult because of its location.
12.3.2.4 Additional Flaps
If none of the flaps discussed above are suitable, others are available for reconstruction, including the free gracilis muscle flap and the latissimus dorsi flap.
12.3.3 Recipient Vessels
The minimal requirements for recipient vessels are one artery and one vein. If the flap is large or seems to be inadequately drained by a single vein, an additional vein should be connected, preferably to a different drainage system (such as the external jugular). The superficial temporal vessels (Fig. 12.10a) and the facial vessels (Fig. 12.10b) are the closest ones available for defects in the anterior or middle skull base. They should be dissected from distal to as proximal as possible so that the caliber of the vessels is wide enough to match those of the flap. If a neck dissection is performed, the exposed vessels of the neck can be used as recipients. For region III posterior skull base defects, the required pedicle length is relatively short, and care should be taken that it does not kink during insetting and anastomosis.
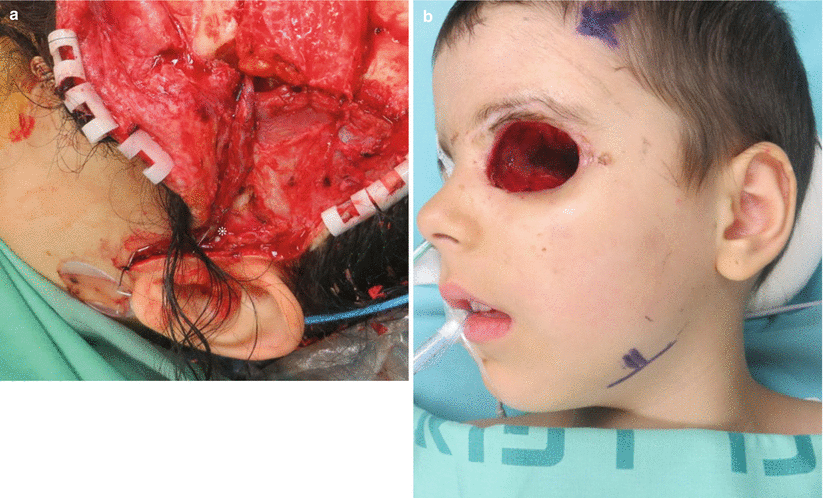

Fig. 12.10
(a) Close-up view on the vascular pedicle anastomosed to the superficial temporal vessels. To achieve a larger caliber, the vessels must be dissected caudal to the tragus. An implantable Doppler was used in this case; its wires and cellulose cuff engulf the vein (asterisk). (b) Preoperative marking of the facial artery and vein at the mandibular rim. They are easily palpable or Dopplered at this area
12.4 Postoperative Protocol and Flap Monitoring
The first 5 days after microsurgical reconstruction are considered critical for flap survival and recovery from the skull base surgery itself. Many microsurgical teams have developed a protocol to improve flap survival and increase the chances of early detection of flap failure:
1.




Flap monitoring every hour for the first 48 h, every 2 h for the next 48 h, and every 4 h for another 24 h.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








