and Ziv Gil2
(1)
Division of Otolaryngology Head and Neck Surgery and Maxillofacial Surgery, Tel Aviv Sourasky Medical Center, Tel Aviv, Israel
(2)
The Head and Neck Center Department of Otolaryngology Head and Neck Surgery, Rambam Healthcare Campus, Haifa, Israel
Keywords
Imaging skull baseImaging skull base pathologyImaging diagnosis of skull base pathologyDifferential diagnosis of skull base pathologyMRI and CT of anterior skull baseImagingIn recent decades, technological advances in clinical diagnosis, preoperative imaging, neurophysiological monitoring, intraoperative microscopy, and multispecialty surgery employing minimally invasive techniques and guidance have advanced the frontiers of the management of skull base pathologies. Current therapeutic techniques include surgical resection, radiation therapy, and chemotherapy. For a successful therapeutic outcome, accurate identification of skull base pathology and care in the subsequent planning of treatment and posttreatment therapy are essential. In all these instances, the choice of imaging modalities and the implementation of this diagnostic entity become critical in properly dealing with patients afflicted with skull base pathologic entities. Imaging is key for assessment, as this region is difficult to evaluate clinically. Imaging allows preoperative tumor staging, identification of characteristics that can lead to the correct diagnosis, and determination of local tumor extent and invasion. It can provide a road map while also determining the surgical approach and evaluating for tumor recurrence.
The objective of this chapter is to present the newest and most effective imaging modalities in current use to establish diagnosis and guide the chosen therapy and subsequent follow-up of patients with skull base pathology. The salient advantages provided by the various imaging modalities are discussed in relation to the most common pathologic entities.
2.1 Imaging Modalities
In the area of the skull base, a bony plate separates the intracranial compartment from the infratemporal/extracranial compartment, and pathologies may arise in this bony separation or in the intracranial compartment or extracranial compartment, so evaluation with both CT and MRI is needed to approach these pathologies. Furthermore, orthogonally oriented imaging is needed to provide a three-dimensional (3D) understanding of the pathology and the regional anatomy giving rise to the pathology. The following sections list the imaging modalities that need to be employed and summarize their purpose and advantages.
2.1.1 Imaging Approach and Protocols
The efficacy and quality of information provided by radiologic assessment depend primarily on the way in which the images are acquired. For example, depending on the plane of acquisition, the slice thickness, and the presence or absence of contrast, a computed tomography (CT) image can vary greatly in its utility to the radiologist and the rest of the team assessing a potential sinonasal cancer patient. Described below are the current standards for imaging the paranasal sinuses. Occasionally these vary depending on the clinical situation (e.g., contrast is rarely used in patients with neutropenic fever being assessed for acute sinusitis), but for the most part they are fairly consistent and reliable.
2.1.2 Computed Tomography
Given its superior bony resolution, CT is typically the best imaging modality for evaluation of the regional delicate bony anatomy and the mucosal changes in the presence of inflammatory paranasal sinus disease. It is also superior in the detection of osseous involvement by pathology, particularly periosteal reaction and bony erosion. CT can help differentiate between benign and aggressive osseous involvement. Particularly when IV contrast is utilized, CT can also provide excellent soft-tissue information, although it is inferior to the soft-tissue contrast resolution provided by MRI.
Images are acquired in the axial plane, typically with 0.5 mm (maximum) slice thickness. The field of imaging should start above the skull base structures and include the entirety of the paranasal sinuses and nasal cavity, orbits, and portions of the middle and anterior cranial fossae. Coronal and sagittal reformatted images are reconstructed from the axial imaging acquisition. If derived from the thin-section source images, these reformatted images should demonstrate excellent spatial resolution, essentially indistinguishable from the axial source images. Coronal images represent the optimal plane for endoscopic correlation. Sagittal planes are very helpful in improving the 3D conceptualization of the regional morphology, but when these images are used, they should always be correlated with an additional orthogonal plane (a coronal or axial plane image) to ensure the accuracy of the orientation. The application of multiplanar reconstruction and the use of crosshairs for localization purposes are especially helpful.
If contrast administration is contemplated, one should first consider performing an MRI examination. Intravenous contrast is usually intended to improve soft-tissue resolution, but the soft-tissue resolution provided by MRI is superior for this purpose, and the radiation dose received with CT is avoided.
2.1.3 Magnetic Resonance Imaging
MRI provides excellent soft-tissue contrast resolution. It offers multiplanar capabilities and does not involve ionizing radiation, a particular advantage when imaging children or women of childbearing age. MRI allows optimal differentiation between sinus mucosal disease and sinus secretions from sinonasal masses. Occasionally, MRI can show early marrow involvement indicating osseous invasion prior to bony erosion.
Imaging is usually performed with a standard head coil, but a surface coil can be employed if necessary. Axial and coronal planes are acquired, with precontrast and postcontrast T1 (with and without fat suppression), noncontrast T2, and noncontrast short T1 inversion recovery (STIR)—a fat-saturated fluid-sensitive sequence—being the standard protocol for evaluation. High resolution with a small field of view and a maximum slice thickness of 3 mm is employed. Intravenous gadolinium is used for postcontrast imaging and helps to better delineate the mass, its extent, and its intracranial involvement; improved tumor-to-soft-tissue contrast is attained with fat suppression. Non-fat-suppressed T1-weighted sequences aid in the identification of perineural spread and vascular involvement by detailing effacement of the normal perineural and perivascular fat planes.
High-resolution imaging of the paranasal sinuses provides a combination of exquisite spatial and contrast resolution. At our institution, isotropic 3D constructive interference in the steady state (CISS) and volumetric interpolated brain examination (VIBE) sequences are performed before and after contrast administration, with evaluation in the axial, coronal, and sagittal planes. An isotropic three-dimensional high-resolution STIR sequence is also performed. This technique provides improved delineation of the mucosal surfaces and often suggests more accurately the margins of sinonasal masses. The high-resolution 3D MRI approach has the added advantage of depicting the relationship of pathology to small anatomic structures including nerves and vascular structures, which are not readily depicted on conventional MRI, allowing for investigation of involvement or distortion by sinonasal malignancy. A high-resolution STIR sequence is employed to assess the T2 signal characteristics of any lesions being evaluated.
2.1.4 Cross-Sectional Angiographic Imaging
Detailed knowledge of the vascular anatomy of the anterior skull base is vital for successful surgery in this region. Preoperative angiographic imaging can help to modify surgical management and alter the surgical approach. In the setting of a highly vascular tumor, CT angiography (CTA) or MR angiography (MRA) can provide information about which vascular pedicles are supplying the tumor, thus guiding preoperative embolization. Anterior skull base tumors may encase or displace arterial structures, including the anterior cerebral arteries and the branch vessels. This knowledge may modify the surgeon’s approach or treatment plan. MRA can be considered when dealing with a vascular lesion or a pathologic process affecting or in relationship with the regional vascular morphology. MRA is an excellent noninvasive means to obtain information about the vascular supply to pathology and to display vascular relationships that may be critical for the surgical approach. MRA does not employ contrast, an advantage for patients with compromised renal function or contrast allergy. MR imaging also does not administer any radiation, an additional advantage over CTA, but CTA provides higher resolution than MRA, relaying important bony detail and vascular anatomy that can aid in surgical guidance.
2.1.5 Positron Emission Tomography
PET is generally reserved for staging and follow-up of sinonasal carcinomas, but it can also be useful in the workup of an unknown primary tumor. Though PET has poor spatial resolution, combining it with simultaneous CT imaging will compensate for this issue. Therefore, a PET/CT study is the preferred evaluation for staging and follow-up. PET/CT can be very helpful in differentiating between recurrence and posttreatment changes.
2.2 Imaging for Initial Diagnosis and Staging
2.2.1 CT Scans
CT scans are the most readily available cross-sectional imaging modality. CT provides the best bone morphologic resolution and the first “glimpse” into the differential diagnosis of the pathology being dealt with. The evaluation should focus on the integrity of the bony architecture and the presence of bone erosion within the sinus morphology, as well as the perimeter bony outline defining the borders of the nasal cavity and paranasal sinuses. Several questions are to be answered:
Is there soft-tissue pathology?
Where exactly is it?
What is its influence on the bony morphology?
Is there extension beyond the boundaries of the nasal cavity and paranasal sinuses?
Important areas assessed by CT scans in defining the extent of pathologic extension include the cribriform plate and planum sphenoidale, the fovea ethmoidalis, the lamina papyracea, the frontal maxillary and sphenoid sinus borders, and the internal bony framework of the sinuses, which is vulnerable to direct invasion and/or destruction. Should the soft-tissue or bony pathology penetrate the paranasal sinus boundaries, the final step is to assess the extent of “invasion” into the orbits or the intracranial compartment through skull base foramina and other possible pathways of spread, including the pterygomaxillary fissure and pterygopalatine fossa. CT is also best used to initially assess bony lesions such as benign fibro-osseous lesions, as well as primary malignant bone tumors. Occasionally, CT can determine the specific diagnosis. CT frequently can differentiate between aggressive bony destruction in the setting of malignancy and osseous remodeling.
2.2.2 MR Imaging
Having identified the pathology on the CT examination, its etiology and its precise extension may still be in question. In this instance, MRI can provide significant additional help. The soft-tissue resolution provided by MRI may be able to further isolate the etiology of the pathologic process. It may distinguish between the various inflammatory pathologies and may be able to isolate a specific neoplastic entity. The use of contrast and fat suppression could significantly improve the accuracy of assessing subtler perineural spread of tumor or intraorbital invasion and may demonstrate invasion of intracranial structures.
2.2.3 PET/CT
In initial diagnosis, PET/CT has little utility except in the case of an unknown primary neoplasm, as in patients with cervical lymph node metastases in whom a primary tumor cannot be detected on physical exam or other conventional imaging modalities. It is uncommon for sinonasal malignancy to be detected in such a way. Most of these neoplasms have a pharyngeal, hypopharyngeal, laryngeal, or tonsillar origin. A small percentage of these tumors are sinonasal, however, and PET/CT has a high sensitivity for detecting such lesions. The detection of residual or recurrent neoplasm in the patient after surgery also can be significantly aided by this modality.
2.2.4 MRI (Standard Imaging)
Compared with CT, MRI has the advantages of multiplanar capabilities and improved soft-tissue contrast. Vessels and their relationship to the tumor are readily demonstrated by MRI without intravenous injection of contrast material. The disadvantage of MRI is its poorer availability, especially in developing countries where many head and neck cancers are more prevalent than they are in the United States. MRI is also not as good as CT in evaluating osseous detail, although MRI can evaluate for early marrow edema and marrow involvement at the skull base prior to osseous erosion. The use of contrast agents has greatly improved the sensitivity of MRI.
Many tumors of the head and neck have intermediate rather than high signal intensity on T2-weighted images, owing to high cellularity and decreased free water. In the epidural space, these tumors become difficult to differentiate from brain parenchyma. Van Tassel and Lee (1991) looked at noncontrast MR images of sinonasal tumors and found that in six of eight patients, the margins of the epidural component of the sinonasal tumor could not be assessed accurately. After administration of contrast material, however, all eight tumors enhanced and could be clearly delineated. The physiology of dural enhancement is currently debated between reactive dural changes and tumor invasion. Recent studies show that the pattern of enhancement is more indicative of tumor involvement than the actual presence of enhancement itself. Ahmadi and coworkers (1993, 1994) showed that dural invasion is present if there is no hypointense zone between the enhancing dura and the tumor on T1-weighted images. These studies also showed that dural invasion corresponded to a discontinuous band of dural enhancement. These and other studies have proposed that a linear pattern of enhancement represents reactive changes in the dura due to adjacent tumor, rather than to tumor invasion.
2.3 Dural, Perineural, and Venous Sinus Invasion by Skull Base Tumors
Surgical resection of skull base tumors, though offering potential cure, may involve significant morbidity, including cranial nerve dysfunction. The value of aggressive surgical resection must be weighed against the natural history of the neoplasm. Intracranial extension is an important prognostic issue and may dictate the treatment of skull base tumors. If the tumor has progressed to the point that negative margins are not attainable, curative or palliative treatment with radiation therapy for malignant tumors is often recommended; surgery may still be used for debulking large tumors. The extent of tumor invasion thus dictates the surgical approach and has significant prognostic implications.
2.3.1 Dural Invasion
Dural invasion by tumor also guides the surgical approach. If dura is to be excised, preparation for pericranial flaps or fascial grafts is important. In a retrospective review of 21 patients who underwent craniofacial reconstruction for anterior skull base tumors, the patients who had dural involvement had a 22 % survival rate after 3 years, whereas patients without dural involvement had a survival rate of 83 %. McIntyre et al. (2012) evaluated MRI findings that correlated with sinonasal tumor involvement.
Several MRI features indicate dural involvement:
Discontinuous band of dural enhancement
Focal, nodular dural enhancement
Dural thickening of more than 2 mm (Fig. 2.1) and pial enhancement
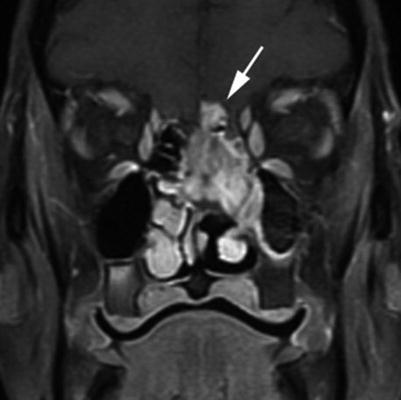
Fig. 2.1
Adenocarcinoma with intracranial invasion. This postcontrast coronal T1 fat-saturated MR image shows an enhancing ethmonasal mass, which extends through the cribriform plate. There is adjacent prominent nodular dural enhancement (arrow), with loss of the hypointense zone, corresponding to dural involvement
Loss of the hypointense zone between enhancing tumor and brain parenchyma on postcontrast coronal T1
Perineural invasion was shown to correlate significantly with increased mortality due to tumor and local or regional tumor recurrence.
2.3.2 Direct Extension and Distant Metastasis
Two routes for distant metastasis are along vascular channels or via neurotrophic spread. Involvement of cranial nerves has been shown to be a poor prognostic sign, and vascular involvement is most likely associated with a poor prognosis.
In a recent study of squamous cell tumors of the temporal bone, patients who had carcinomatous involvement of the dura mater did not have improved survival rates with surgical management. Patients whose tumors invaded through the dura died within 5 months of attempted resection, whereas patients whose disease did not extend intracranially had approximately a 50 % 5-year survival rate after surgical resection. Before the advent of MRI, invasion of the dura, venous sinuses, and cranial nerves was assessed during surgery, but the degree of soft-tissue resolution achieved with MRI has enabled improved delineation of tumor extent. An accurate prediction of invasion of these structures with MRI provides hope for a clearer prognosis at presentation and more appropriate treatment decisions. MRI has the advantages of multiplanar capabilities and better soft-tissue contrast than other imaging techniques. With the development of MR contrast agents, the value of MRI as a diagnostic tool for skull base neoplasms has further increased.
2.4 Pathology
2.4.1 Infection and Inflammation
Sinonasal infectious processes can ultimately extend intracranially, considering that only a thin, bony plate separates the two compartments. Intracranial spread of infection most commonly arises from frontal sinusitis (into the anterior cranial fossa) and can have devastating consequences; close attention should be paid for the subtle signs that may be seen on both CT and MRI. The potential manifestations of intracranial spread of infection include subdural or epidural empyema, intraparenchymal abscess, cerebritis, meningitis, mycotic aneurysms, vasculitis, cavernous sinus thrombosis, or venous or arterial infarcts secondary to vascular thrombosis. Infection can spread via direct extension, either directly through the bone or in a perivascular manner.
MRI is the imaging modality of choice if intracranial spread of infection is suspected, because of its exquisite contrast resolution, allowing for improved detection and characterization of pathologic complications. CT, however, can usually detect extrasinus spread and is frequently the first test ordered. On CT, subtle osseous erosions at the skull base may be the earliest indication, although this erosion is not always seen. Rim-enhancing subdural or epidural empyema or parenchymal abscess may develop and can be seen on postcontrast CT. On MRI, these fluid collections are frequently T2 hyperintense and can be of variable T1 signal. On post-gadolinium T1-weighted sequences, there is rim enhancement (Fig. 2.2). The most important sequence to evaluate for pyogenic abscess or empyema formation is diffusion-weighted imaging (Fig. 2.2d) which will show intrinsic diffusion restriction. Acute infarcts or hemorrhagic infarcts in the setting of venous thrombosis can also manifest. On postcontrast CT, CT venogram, or MR venogram (MRV), filling defects in the cavernous sinuses, with enlargement of the superior ophthalmic veins, should raise the suspicion of cavernous sinus thrombosis. During the subacute thrombus phase, however, venous thrombus may not be readily detected on time-of-flight MRV owing to intrinsic T1 shortening. Meningitis is frequently not seen on CT imaging, although mild ventricular or subarachnoid space enlargement can be the earliest sign. On MRI, ventricular enlargement can also be seen in addition to increased meningeal enhancement. As the infection progresses, inflammatory extra-axial exudates can be seen.
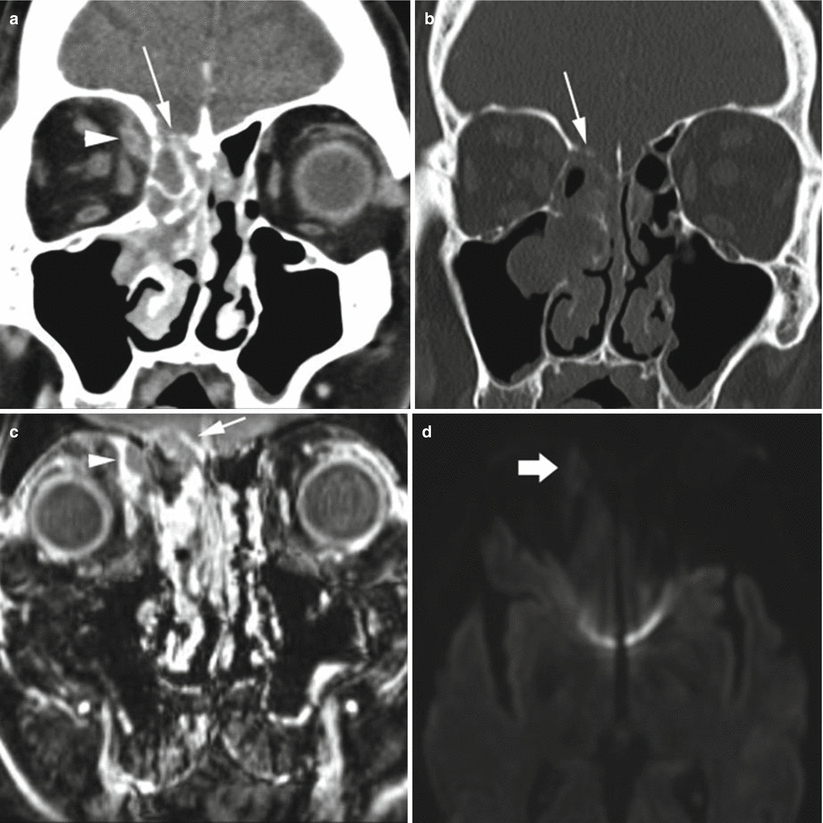

Fig. 2.2




Acute sinus infection. On coronal postcontrast CT image at the level of the orbits in soft tissue (a) and bone window (b) algorithm, soft tissue fills the right nasal cavity, with erosion of the right cribriform plate and fovea ethmoidalis (arrow) and a rim-enhancing right orbital subperiosteal fluid collection representing abscess (arrowhead). On coronal fat-saturated T1 postcontrast MRI sequence at the level of the orbits (c), there is right nasal and ethmoid enhancing mucosal disease, with rim-enhancing abscess in the medial right orbit (arrowhead), as well as right anterior skull base epidural abscess (short arrow). On axial diffusion-weighted imaging (d), the right orbital fluid collection shows mild diffusion restriction (thick arrow), confirmed on the apparent diffusion coefficient (ADC) map (not shown)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








