Key points
• Skin, fat, muscle, fascia, tendon, and bone can all be differentially carried on vascular pedicles from various donor sites in the provision of vascularized tissue to the face.
• Soft tissue, muscle, and bone flaps will all be reviewed with a focus on their use for facial soft tissue or bony augmentation of the face for both aesthetic and reconstructive purposes.
• Each particular flap can be tailored exactly, depending on the defect or lack of tissues that is being addressed, in a “like with like” manner.
• Technical as well as aesthetic considerations to enhance outcomes in these cases will be emphasized.
Introduction
Microsurgical techniques may bring a plethora of vascularized tissue types to the facial skeleton and soft tissue structures when local tissues, grafts, alloplasts, or other materials are insufficient. Thus microsurgery offers the unique ability to truly replace “like with like” tissues. Common etiologies of missing facial tissues include trauma, cancerous or benign tumors, and congenital conditions, among others. In such cases, microsurgery is a key tool in the armamentarium of the plastic surgeon in achieving an equally functional and aesthetic outcome. The tissue provided can be tailored to the exact need of the defect, the deficient tissue, and the patient as a whole. These tissues may include bone, muscle, fascia, tendon, fat, and skin in variable configurations, based on the vascular pedicles on which they are carried. The major disadvantage of microsurgical techniques is donor site morbidity, with each donor site having unique characteristics. However, the majority of donor sites, especially with the increasing use of perforator flaps, tolerate the techniques very well.
As is well-known in the plastic surgery community, the line between aesthetic surgery and reconstructive surgery is always blurred. In many cases, use of microsurgical techniques to obtain distant, vascularized tissue allows for not only a more functional reconstruction but also a more aesthetic reconstruction compared with that of nonmicrosurgical techniques. In certain circumstances, the indication for microsurgical augmentation of the head and neck may be a primarily cosmetic deformity, as in the use of a buried parascapular flap in Parry-Romberg syndrome. In others, microsurgical tools are employed in an effort to aesthetically improve the outcome of a particular procedure and to obtain functional benefits. An example is the implementation of an immediate medial sural artery perforator flap for postparotidectomy defects, not only to provide a layer of vascularized tissue to prevent aberrant nerve regeneration but also to obviate the facial contour abnormalities that are common after parotidectomy alone. Microsurgical flaps play a primarily reconstructive role in the face and facial skeleton in select cases, such as mandibular reconstruction with an osteocutaneous fibula flap after trauma or oncologic resection. However, even in these contexts, the surgeon should have an eye on the ultimate aesthetics of such reconstructions, whether that be achieved primarily or in multiple subsequent stages. Facial composite tissue allotransplantation (CTA) represents the ultimate intersection of aesthetic and reconstructive facial augmentation. Facial CTA has revolutionized the field of facial reconstruction, with the entire facial unit, including bone, muscle, fascia, and skin, provided as a single vascularized flap in the most severe facial deformities. In this manner, an unparalleled aesthetic facial reconstruction is achieved.
Throughout this chapter, we will demonstrate the confluence of aesthetic and reconstructive techniques in discussing flaps for microsurgical augmentation of the facial soft tissues and bony skeleton, including their indications and the important aesthetic considerations in their utilization.
Patient evaluation
History
A complete history must be taken for any patient under consideration for microsurgical augmentation of the face. To begin, the etiology of the expected or actual facial defect should be ascertained. Often, patients are referred to the microsurgeon with an already known diagnosis. However, in the instances when the plastic surgeon will be the first person to evaluate the patient, the surgeon must be able to diagnose a range of etiologies, which will be discussed later. The inciting symptoms, their chronicity, location, and progression, as well as any associated issues should be thoroughly investigated.
Next, full medical and surgical histories are reviewed. Patients with significant multisystem comorbidities that preclude their ability to withstand general anesthesia for a lengthy intraoperative time are not good candidates for microsurgical procedures. Any comorbidity should be medically optimized before proceeding. Special attention should be given to any history suggesting a hypercoagulable state. This includes, but is not limited to, a history of deep vein thrombosis; autoimmune conditions, such as lupus; multiple failed pregnancies in women; and prior microsurgical failures. Any concerning past issues should be further investigated and addressed with laboratory work and/or consultation with a hematologist because hypercoagulability can compromise an otherwise technically sound microsurgical procedure. Patients with a bleeding diastasis should be assessed to ensure that they are able to safely tolerate the postoperative antiplatelet and anticoagulant therapy that may be administered.
Prior history of radiation to the face should be ascertained because resultant tissue fibrosis and impaired wound healing will impact dissection in the area. The surgeon must also assess for prior surgical interventions undertaken in the head and/or neck as well as any potential donor sites. Scarring of recipient sites in the head and neck region can influence dissection as well as availability of recipient vessels for anastomosis. Donor site scarring from prior surgery may lead the surgeon to select another area offering similar tissues for transfer.
Medications and allergies are also assessed—specifically, any medications with a hypercoagulability profile, including oral contraceptives and selective estrogen receptor modulators, among others. These should be held for at least a week before the surgical procedure. Patients who are active smokers should stop smoking for at least 4 to 6 weeks preoperatively and for at least 2 weeks postoperatively. Although smoking has not been shown to increase the risk of microsurgical failure, it may increase risk of wound healing complications, especially those involving the donor site, through the mechanisms of platelet aggregation and microvascular vasoconstriction. Finally, the patient’s occupations and hobbies should be reviewed. This information will assist in selecting a donor site that will tolerate the procedure well, with the least possible adverse impact on the patient’s daily life.
Differential diagnosis
The differential diagnosis of the various conditions that necessitate microsurgical facial augmentation can be categorized by the affected facial tissues. This differentiation becomes apparent upon gathering a complete history, as described previously, and through a meticulous physical examination, as discussed later. Missing or deficient facial tissue layers may occur in isolation, such as in a full-thickness skin resection of a basal cell tumor, or in combination, such as in a trauma-related composite mandibular defect that includes skin, muscle, and bone.
The soft tissue of the face begins superficially as the skin and superficial fat, below which are the superficial musculoaponeurotic system (SMAS) fascia and deeper fat compartments. Skin and soft tissue loss requiring microsurgical augmentation may occur as a result of surgical resection, trauma, congenital, or idiopathic causes. Advanced locally aggressive benign or malignant tumors in the face may result in large defects that are insufficiently reconstructed with local tissues or other methods, mandating distant tissue be brought in for reconstruction. Alternatively, specialized areas of the face, such as the eyelids, nose, and mouth, may require distant tissue for reconstruction even with smaller defects due to their specialized function and paucity of local tissues available for reconstruction. In the setting of prior or future radiation, local tissues will be poorly suited for reconstruction, and microsurgical tissue transfer may be required for defects that otherwise may be amenable to local tissue rearrangement.
Similarly, trauma can result in complex and substantial soft tissue defects of the face, which, due to size and/or location, are best reconstructed with microsurgical techniques. Importantly, with traumatic injuries, there exists a zone of injury surrounding the area of maximum trauma that impacts vascular inflow. The surgeon should ensure that the recipient vessel of choice for any free tissue transfer is outside of this zone of injury, and this may necessitate vein grafting.
Congenital causes of facial soft tissue deficiency include various forms of hemifacial microsomia and the associated syndromes, in which the facial soft tissue as well as bone can be hypoplastic. The most common idiopathic causes of facial soft tissue loss or deficiency are linear scleroderma and Parry-Romberg syndrome, which are slowly progressive and usually unilateral conditions of unknown cause. The severity of such deficiencies with these causes is variable. In significant cases, microsurgical augmentation with soft tissue flaps offers the advantage of greater volume with improved stability and longevity compared with augmentation with alloplastic or autologous grafting techniques.
The facial muscles are invested by the SMAS layer, lying superficial to the facial skeleton. Microsurgical augmentation of the facial musculature is generally undertaken to restore lost facial nerve function in the setting of native facial muscle fibrosis after a prolonged period of denervation. Etiologies of facial nerve dysfunction include idiopathic causes, such as Bell’s palsy, nerve tumors or involvement of adjacent tumors, most commonly of the parotid gland, as well as trauma and congenital cases as in Möbius syndrome.
Osseous defects or deficiencies of the facial skeleton can arise from a variety of etiologies. The most common include trauma; malignant or locally invasive tumors, the most common of which are squamous cell carcinoma and ameloblastoma, respectively; osteoradionecrosis; and osteomyelitis. Given the deep location and close investing relationship of bone to other facial tissues, isolated bony defects are less common. Often, resection of tumors, infection, or osteoradionecrosis will also involve resection of the skin, mucosa, or musculature, creating composite defects that are best treated with free tissue transfer.
Aging will also result in facial soft tissue loss, specifically loss of the deeper fat compartments, as well as bony resorption of the facial skeleton. However, microsurgical augmentation of involutional fat deflation or osseous changes associated with aging are more appropriately treated with fat grafting or other means, rather than microsurgical techniques.
Physical examination
The physical examination should first focus on the actual or potential defect to be addressed microsurgically. To begin, if a defect or deficiency already exists, it is examined to determine its extent and the layers of the face, from the skin to bone, that are involved. This includes an intraoral examination. The quality and laxity of facial tissues surrounding the real or future defect are assessed to determine whether they are sufficient for reconstruction. The presence or absence of stigmata of prior radiation or facial scars are determined. A full cranial nerve examination should always be performed, with a focus on the ability of the patient to close the eyes fully, smile symmetrically, and achieve oral competence. This is especially important when assessing patients as candidates for microsurgical facial reanimation with free muscle transfer. The neck should also be examined, with special attention given to any prior scars that may influence recipient vessel selection.
Once the recipient site has been addressed, potential donor sites must be examined. These will vary, depending on the needs of the defect/deficiency; however, the most common are the upper and lower extremities as well as the back. The donor sites should be examined for prior scarring, tissue quality, pliability, laxity, and color for the best match for the needs of the recipient site. In the extremities, it is imperative to assess the distal vasculature to ensure that harvesting of the donor vessel pedicle will not compromise perfusion to the remaining extremity. In the upper extremity, the Allen test should be performed to assess a radial- or ulnar-dominant extremity, which would preclude the use of a radial or ulnar forearm flap, respectively. In the lower extremity, the dorsalis pedis and the posterior tibial arteries should be palpated to ensure good perfusion of the extremity. Lack of palpable pulses requires further investigation with Doppler ultrasonography or more invasive imaging.
Imaging or other preoperative diagnostic evaluations
Various imaging modalities can assist the surgeon in the evaluation of patients for microsurgical augmentation of the face. While assessment of the facial soft tissues is best achieved through clinical examination, imaging of the recipient site(s) in the head and neck region may help define the defect or deficiencies to be addressed and establish the vascular status of potential recipient vessels. Three-dimensional (3D) imaging has proven to be a useful adjunct to predict volume deficiencies or differences in unilateral cases. This can help plan the amount or configuration of donor tissue required, in turn, will assist in donor site selection, dissection, and inset into the recipient site. Magnetic resonance imaging (MRI) also has utility in assessing the location and volume of facial soft tissue deficiency. In cases of planned microsurgical free muscle transfer, electromyography and nerve conduction studies may be useful in assessing the status of the facial nerve and musculature to determine patient candidacy. Osseous defects are best appraised with Panorex plain films or computed tomography (CT). CT or magnetic resonance angiography (MRA) can assist in assessing the availability of recipient vessels for anastomosis.
Importantly, imaging of the donor site gives the surgeon valuable information by delineating flap perforator and pedicle orientation, location, and course, and helps determine adequate distal perfusion with extremity flaps—for example, ensuring lack of a dominant peronea magna, which would result in a dysvascular leg with fibula flap harvest. Formal angiography is the gold standard; however, this is rarely necessary, given its invasive nature, except in cases where noninvasive imaging cannot be performed or is inconclusive. CT or MRA are noninvasive modalities to assess the perforator and pedicle vascular anatomy of flap donor sites. Computed tomography angiography (CTA) is quicker and less expensive but may be precluded in patients with renal disease or other contraindications, in which case MRA is a second-line choice. Routine preoperative imaging, in the form of CT or MRA, is generally employed for bony or fasciocutaneous flaps and less commonly for muscle flaps, to precisely ascertain the spatial relationships between the pedicle and the perforators. Regardless of the flap type, imaging is prudent if surgical intervention had been previously undertaken in the region to ensure that damage to the perforators or the pedicle of the planned flap has not be compromised.
Indications and contraindications
The indication for a microsurgical approach to facial reconstruction is lack of facial soft tissue and/or bone that cannot be adequately or safely replaced with local tissues, grafts, alloplasts, or other materials. Microsurgery has the advantage of offering distant, diverse, and vascularized tissues on a vascular pedicle in these cases. Often, these are primary cases of tissue loss due to trauma, locally aggressive benign or malignant tumors, or congenital conditions (e.g., Parry-Romberg syndrome). However, secondary microsurgical facial augmentation may also be indicated in certain scenarios. These commonly include primary reconstruction with other methods that proved insufficient over time, especially when the primary extirpation was combined with radiation therapy. In general, bony defects greater than 6 cm in length will benefit from microsurgical tissue transfer compared with traditional bone grafting. Furthermore, central bony defects of the mandible that are even a few centimeters in length are better served with vascularized bone. With missing muscle function due to muscle fibrosis from prolonged facial nerve palsy lasting greater than 18 to 24 months, the best approach is with microsurgical muscle transfer because muscle grafts will not survive without direct vascularization. Missing facial soft tissues of moderate to significant dimension are often better reconstructed with microvascular tissue transfer because they are not limited by variable resorption, as with fat grafting. Importantly, in the face of radiation, vascularized tissue offers distinct advantages compared with grafts and alloplasts, which have complications, such as poor take, extrusion, and infection.
Contraindications to microsurgical approaches in facial augmentation are similar to those for all microsurgical procedures. To begin, the overall condition of the patient must be considered. Although advances in microsurgical principles have increased the efficiency of these procedures, patients must be physiologically able to tolerate prolonged general anesthesia to be candidates. Furthermore, the risk of systemic thrombosis should be assessed. Patients with a history of real or possible thrombotic events, such as deep vein thrombosis, those with multiple failed pregnancies, and those prone to thrombotic events, such as those with factor V Leiden deficiency, lupus, or sickle cell anemia, are at higher risk of vessel thrombosis and microsurgical flap failure. Consultation with a hematologist, specific testing, and consideration of different techniques for facial augmentation in these patients are advised.
Next, recipient and donor site assessments must be made in determining patient candidacy. The recipient site is assessed in terms of potential vascular inflow for the microsurgical flap. A recipient vessel of adequate location, inflow, size match, and expendability must be present. If such a vessel is not present, microsurgical techniques are not necessarily precluded; however, adjunctive measures, such as vein grafting or arteriovenous loop creation, may be necessary. Similarly, availability of donor sites must be ensured in determining a patient’s candidacy for microsurgical facial augmentation. A donor site offering a match in terms of quality and amount of tissues available, vessel caliber and length, and minimal and acceptable associated morbidity must be present to proceed.
Preoperative planning
Preoperative planning begins with a thorough history and physical examination, as discussed previously. The types and dimensions of missing tissues and the areas of the face to be augmented are first assessed. Functional and aesthetic goals are set with the patient. Once these aspects are determined, an appropriate donor site must be selected. As discussed, the ideal donor site is one that provides the exact amount and types of tissues desired, with a vascular pedicle that is of sufficient caliber and length for tension-free anastomosis to the recipient inflow vessels. Moreover, the donor site should be tolerant of the procedure and with the least possible morbidity with regard to form and function. Although no donor site may be ideal in any given clinical scenario, the one closest to meeting these criteria should be selected.
Preoperative imaging plays a significant role in planning microsurgical augmentation of the face, as discussed previously. Furthermore, the intersection of imaging and microsurgery is virtual surgical planning (VSP), which has helped optimize outcomes in complex head and neck microsurgical reconstructions. Primarily employed in cases of bony reconstruction, VSP permits surgeons to conceptualize and modulate any imaged recipient and donor site. As such, the facial skeleton can be adjusted on the basis of the operative plan to better visualize the expected or real defect. Similarly, donor site tissues can be manipulated to determine the dimensions required to adequately address the needs of the recipient site. Moreover, operative models and even cutting guides for bone cuts based on this planning can be printed in 3D for utilization in the operating room. Incorporation of VSP offers the opportunity to maximize efficiency and optimize surgical results in cases of complex, microsurgical facial augmentation. However, a relatively steep learning curve is involved.
Primary operative approach
The general operative approach in microsurgical augmentation of the face is to replace missing or deficient tissues with the best “like with like” match while minimizing donor site morbidity. This should also be accomplished in a manner that minimizes scarring and distortion of the facial recipient site(s) while achieving symmetry and coming as close to the aesthetic ideal as possible. Minimal healthy tissues should be excised from the face.
As an example, in unilateral Parry-Romberg syndrome, the operative approach will generally involve elevating the facial skin overlying the deficient areas through a facelift incision and restoration of exact volume via an often-buried fasciocutaneous flap, such as a parascapular flap from the subscapular vascular system of the back. The chosen incision that is necessary for access will eventually result in concealed recipient site scarring. The parascapular flap provides adipose tissue and skin, which can be deepithelialized, as needed, and matches the deficient tissues in a “like with like” fashion. Anastomosis can be performed within the operative field of the access incision to the superficial temporal vessels, which are best taken to their trunk to avoid issues with vasospasm. The donor site also carries minimal morbidity and a well-hidden scar. Inset of the flap to the recipient site should be performed meticulously to accurately and precisely fill in the deficient or missing areas. Percutaneous bolster sutures may help in this regard. Flap placement should also be done taking into consideration potential tissue ptosis, which will have the effect of aging in the face. The soft tissue flap should be secured to stable facial structures, such as the periosteum of the zygoma or the inferolateral orbital rim, to prevent such premature descent. Any procedure near the lower eyelid mandates stable flap securement such that descent will not pull down the eyelid and result in ectropion.
A case of free muscle tissue transfer for microsurgical augmentation will often involve a similar access incision and recipient vessels to minimize facial scarring. A donor site with an expendable muscle that will not significantly alter residual function is chosen, with the gracilis, pectoralis minor, and serratus muscles being the most common. The muscle should be inset at its natural resting tension for appropriate motion with contraction. The line of muscle pull should be designed with consideration of the desired facial movement to be achieved. For example, in cases of smile restoration, the donor muscle should be inset with a line of pull along the zygomaticus major muscle. Nerve coaptation in these cases is performed to the ipsilateral facial nerve trunk, if available; a cross facial nerve graft; or another nearby donor nerve, such as the masseteric nerve.
Bony microsurgical augmentation requires considerably more dissection for recipient site preparation, whether that involves bony resection or simply exposure of the areas to be augmented. If muscle and soft tissue are to be resected or are missing along with bone, these should be brought in with the flap as well. Many osseous flaps can carry composite tissues and are advantageous in this regard. Recipient site exposure therefore is often more extensive in these cases; however, external scars should be designed to be as inconspicuous as possible and within the borders of anatomic subunits. The best donor sites are those offering expendable cortical and/or cancellous bone with good size and shape match to the bone to be replaced. Donor site morbidity should be minimized as much as possible. The fibula is ideal in this regard, with the iliac crest, scapula, and radius being secondary options. When the donor site is brought to the recipient site, bony apposition of the osseous flap edges to native bone should be maximized. Certain flaps, such as the fibula flap, permit osteotomies to be performed, giving the surgeon great flexibility in shaping the bone to provide the most aesthetic match in terms of projection and shape to the native facial skeleton. Fixation in a load-sharing fashion is most appropriate, especially in nonmandibular reconstruction. Large, unstable, or anterior mandibular defects are better reconstructed with load-bearing fixation.
Depending on the circumstances dictated in each case and the surgeon’s preference, skin paddles are often utilized and remain exposed on the face after microsurgical augmentation. In certain cases, this is necessary because the skin flap is replacing lost native facial skin. In other cases, the majority of the flap may be buried for volume replacement, while a small skin paddle remains to help monitor the flap. Either way, the approach to designing the skin paddle, whether the skin paddle is the only flap component or is part of a composite flap with muscle and/or bone, should take aesthetics into consideration because this will be the most visible portion to the patient. When the plan is for the skin paddle to remain over the long term, a subunit approach, in which the paddle resurfaces an entire subunit, be it on the cheek, chin, or anywhere else on the face, should be undertaken. If the skin paddle is serving a primarily monitoring role, it can be designed to be small and placed along the incision, with a plan for excision at a later date.
Alternative approaches
In select circumstances, when only bone, muscle, or deeper soft tissues are to be replaced, all of the flap can be completely buried under the facial skin flap. In this case, the skin elements of the flap are completely deepithelialized before the flap is placed. The buried flap should be meticulously positioned by using internal sutures or external suture bolsters to precisely replace and fill areas of deficient or missing volume, as described previously. After this is done and the anastomoses are complete, the facial skin flap is simply sutured with the resultant incision line as the only stigmata of surgery. The classic example of this is the utilization of a buried parascapular flap for facial soft tissue augmentation in patients with Parry-Romberg syndrome. However, there is no skin paddle to assist in postoperative flap monitoring with completely buried flaps. Therefore adjunctive means of flap monitoring are required to avoid unrecognized flap compromise or failure. Implantable Doppler probes, placed around the vascular anastomoses and connected percutaneously to a monitor, are well suited for this task. Any change or loss of signal with this device, which may be due to device malfunction or issues with the flap itself, mandates a return to the operating room for exploration. Handheld Doppler devices may be used to examine through the facial skin, but care must be taken to ensure that the transmitted vessels are associated with the flap and not native facial or neck vessels.
As discussed previously, minimal healthy facial tissues, especially skin, should be excised in preparing the recipient site to minimize scarring and maximize the presence of healthy facial tissue. However, the subunit principle may be applied in select cases, although its implementation is still being debated and is dependent on the surgeon’s preference. The subunit dictates that when greater than 50% of a facial subunit is being excised, be it a nasal, cheek, chin, or other facial subunit, then the entire subunit, including adjacent healthy tissues, should be removed with the subunit being reconstructed as a whole. Proponents argue that this results in a more aesthetic reconstruction, whereas others counter that maximizing the presence of native facial skin and tissue will result in great aesthetic value. This may carry even greater weight when considering microsurgical reconstruction in which the distant tissue provided is often a less exact match in terms of color and texture compared with local tissues. However, the surgeon should be familiar with this concept and consider if its implementation will improve the aesthetics of microsurgical facial augmentation in each unique case. The specific techniques to achieve these goals will vary, depending on the defect/deficiency, the area of the face being addressed, and the flap chosen to address the issue at hand.
Optimizing outcomes
The definition of successful facial reconstruction has changed as it continues to evolve in the twenty-first century. During the 1970s when microsurgery was developing, the primary aim was of the flap and its ability to “fill the defect.” With flap success rates currently ranging from 95% to 99%, the focus should no longer be on long-term flap viability but, rather, on improving functional outcomes. Advancements in the fields of microsurgery, craniofacial surgery, and aesthetic surgery have therefore refined the goal to “replacing like with like.” Adhering to these established principles is paramount to optimizing outcomes.
The most important consideration is ensuring the integrity of the alimentary tract, face, and neck. This also includes separation of the tracts from the intracranial contents. Failure to do so would lead to complications, such as orocutaneous fistula, or even life-threatening events, such as carotid artery rupture. Not only is the patient’s ability to feed compromised, but also chronicity of persistent wounds leads to further scarring and disfigurement. Currently, there is no ideal substitute for the oral mucosa, although the best alternative is skin. Pliability of skin, viability, and flap thickness are important aspects, and as such, many have advocated for either radial forearm or medial sural artery perforator flaps as the preferred donor sites. These characteristics also make them popular selections to replace facial skin, particularly that of the cheek. In cases where soft tissue bulk is needed to obliterate dead space or in areas of irradiated wound beds to help augment the integrity of the alimentary tract, the anterolateral thigh is a good option for the donor site because of the ability to include the vastus lateralis muscle.
Sound principles of craniofacial anatomy should be applied when reconstructing the bony craniomaxillofacial complex. The facial bony horizontal and vertical buttresses should be respected. Design of osseous reconstruction should mimic the facial skeletal horizontal and vertical buttresses as closely as possible. They serve not only as pillars of functional loading of the intermaxillary complex but also as powerful anchors in establishing facial contours necessary for appropriate soft tissue draping. Therefore ensuring optimal osseous union between flap and native bone is of great importance. Osteotomy cuts should be designed to maximize surface contact areas. Although other options, such as alloplastic implants and nonvascularized grafts, exist, vascularized bone grafts remain superior with regard to minimal, predictable bone resorption and are ideal in areas with overlying thin soft tissue envelope, particularly the periorbital and zygomatic regions.
Once the skeletal construct of the defect has been deemed adequate, volume deficiency of soft tissue should be assessed. The primary objective is to provide slight volume excess with slight contour overcorrection. Fat grafting and volumizing agents can mask certain select cases of minor skeletal deficiencies. However, it has been noted that serial autologous fat injections do not maintain stable long-term symmetry, particularly in Parry-Romberg syndrome. Siebert et al. reported superior results with microsurgical augmentation through parascapular flaps. The versatility of this flap allows for inclusion of the dorsal thoracic fascial extensions overlying the latissimus dorsi, trapezius, and serratus anterior muscles. These fascial extensions help augment the upper lip, piriform aperture, and malar prominence, allowing for precise contouring of even subtle deformities. Furthermore, Siebert et al. further reported an improvement in proinflammatory gene expression profiles in these patients after microvascular free tissue transfer and thus improved clinical outcomes.
The introduction of aesthetic subunits to facial reconstruction has been championed by some authors, such as Eduardo Rodriguez. Respect for aesthetic unit boundaries will allow for better results. Defects involving greater than 60% of an aesthetic subunit should have the resection extended to the nearest subunit border. In doing so, scars can be positioned in better concealing areas, such as hairlines, eyebrows, and facial shadows. Finally, it is also important that the surgeon uses all that is within his or her armamentarium to optimize results. Local and regional flaps are powerful adjuncts when used in combination with free flaps, reducing larger defects to manageable aesthetic subunits. Furthermore, the use of tissue expanders, strategically placed along the mandible and/or neck, can allow for large cervicofacial flaps to be rotated onto the face, providing the ideal skin to cover the free flaps or to minimize the defects to be reconstructed with free flaps.
Surgical technique
Although there are numerous free flaps that one can consider for head and neck reconstruction, we have limited the discussion of surgical techniques to three “workhorse” flaps.
Radial forearm flap (fasciocutaneous flap)
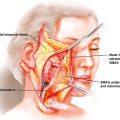
This thin, relatively hairless flap with a lengthy pedicle (20 cm) of large external diameter can be employed as a neurosensory flap, with inclusion of either the medial or the lateral antebrachial cutaneous nerve. After confirming adequate flow to the hand and the digits via the ulnar artery with the Allen test, an appropriate-sized cutaneous flap is positioned and marked on the forearm, with an average skin paddle size measuring 5 to 8 cm in width and 8 to 10 cm in length. The flap is designed slightly more radial to not only center the flap over the radial artery but also include the cephalic vein whose venous tributaries coalesce over the anatomic “snuff box.” The course of the brachial artery and the radial vessels, locations of the medial and lateral antebrachial cutaneous nerves, and the cephalic vein are identified and marked. An incision is made on the ulnar flap margin, often including the deep fascia of the forearm. The distal flap margin is also incised to the radial artery, ligating distal superficial veins, and the proximal flap margin is incised taking great care to avoid injury to the cephalic and basilic veins and to the medial and lateral antebrachial cutaneous nerves, if they are not to be included.
Dissection is then carried in the ulnar-to-radial direction, identifying the palmaris longus tendon as well as the radial border of flexor carpi radialis tendon. An incision is then made alongside the proximal radial margin subfascially until the brachioradialis tendon is identified and retracted laterally. Exposure of the radial artery and venae comitantes is performed while preserving the superficial branches of the radial nerve, which generally lies more laterally in the lateral intermuscular septum.
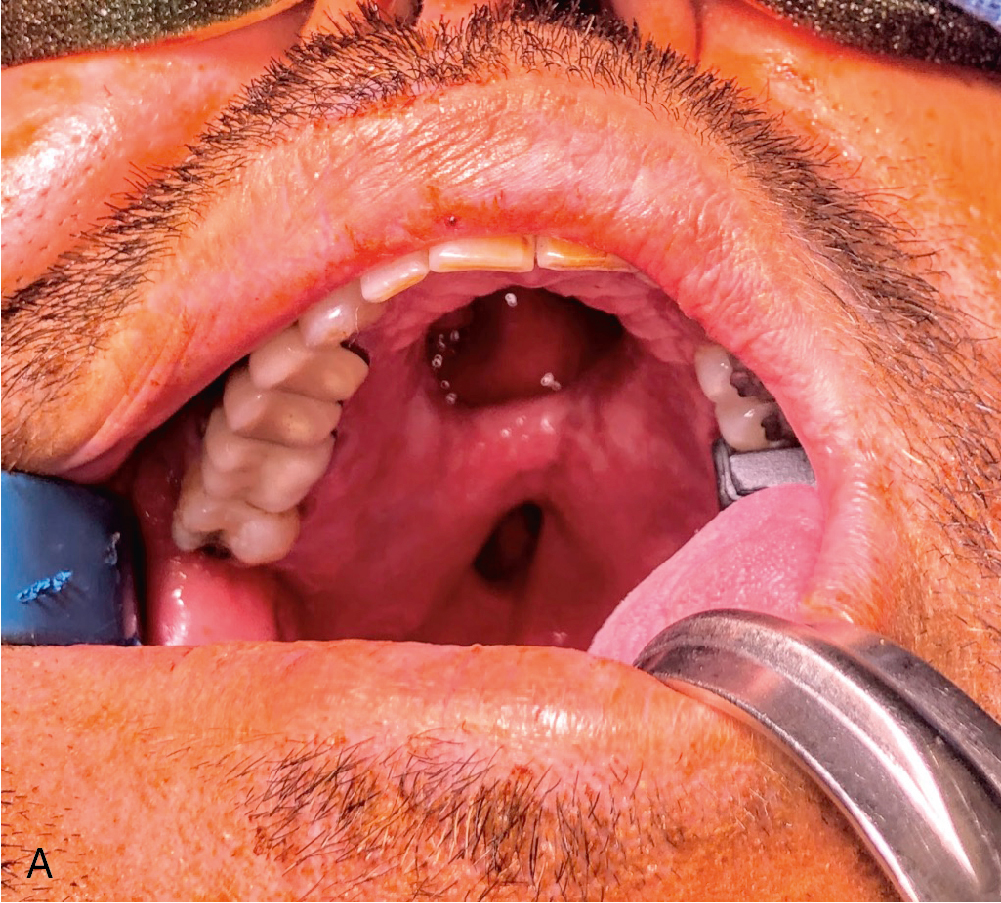
After freeing the nerve, the intermuscular septum and associated radial artery’s venae comitantes and cutaneous perforators are elevated from the radius in the distal-to-proximal direction, clipping branches to deep muscular structures until the desired pedicle length is reached. Proximal dissection can extend until ulnar artery is identified, and the venous dissection can be dissected proximal until the “H” connection, which is often present connecting the deep system of the venae comitantes with the superficial system of the cephalic vein, is reached. The pliability and thin nature of this flap makes it useful in intraoral reconstruction ( Fig. 39.1 A–C).