(1)
Medical Faculty, Acibadem University, Istanbul, Turkey
Abstract
Long lasting success on complex surgical research models depends on meticulous surgery, proper handling of the animal all through the treatment and the careful observation of vital signs. Multiple intravascular catheters at multiple sites are still being used today and technique is named “invasive monitoring”. Modern invasive techniques measures mean arterial pressure, PaO2, PaCO2 a central venous pressure (CVP) values. Cannulation procedures give important information regarding the conditions of the animal despite having certain harmful effects at the micro-circulatory level. Thus, the process of vessel catheterization requires attention and should be reported in experimental methods. The adverse effects produced from the cannulation techniques should always be considered.
Keywords
MicrocirculationModelInvasiveAnimalMonitoringLong lasting success on complex surgical operations depend on meticulous surgery, proper handling of the animal all through the treatment and the careful observation of vital signs. Multiple intravascular catheters at multiple sites are still being used today and technique is named “invasive monitoring”.
Modern invasive techniques measure mean arterial pressure, PaO2, PaCO2 an central venous pressure (CVP) values. Despite numerous benefits, this operation does come with some drawbacks. Most commonly; infections and inflammation can be seen at the insertion site, local pain and discomfort are observed, mispositioning of the catheter tip can occur, wound dehiscence, subcutaneous hematoma, accidental injection of drugs (i.e.; adrenaline) and soft tissue related problems become prevalent. Secondary and late complications include venous and central line thrombosis, pneumothorax, bronchial erosion, vagal reflex activation, and lethal conditions such as embolization and cardiac tamponade can be observed [1, 2, 4–12]. Moreover the formation of arterial emboli is also common at the cannulation site. Today we also know that detaching parts of the thrombus can lead to significant perturbations in flow hemodynamics and capillary perfusion [13].
Thus the creation of a live model is paramount to fully elaborate on the defined risk of thrombus formation and observe the affects of arterial and venous catheterization.
The cremaster muscle flap model is a versatile model that is developed for performing any kind of microcirculatory research of the skeletal muscle. It was first introduced by Baez [14], for in vivo observation of microcirculation under microscopic magnification. This flap is isolated on a single neurovascular pedicle as an island flap [15], and numerous surgical research scenarios can be applied to it [15–18]. During the research, microcirculatory parameters can easily be recorded by intravital observation of the muscle flap, including vessel diameters, red blood cell velocities, capillary densities, leukocyte – endothelial interactions, and endothelial edema index.
Technique
Vessel Cannulation
Vessel cannulation aiming to monitor the vital signs of rats should be performed just before flap dissection. After the induction of anesthesia, and confirming that the animal is pain free, the rat is placed at supine position on wooden plates with all the extremities positioned at abduction and extension. The cervical and inguinal areas of the rat is shaved taking care not to injure the skin and swabbed with povidone-iodine in order to achieve sterility at the areas of surgical intervention. Oblique incisions about 2 cm long are made on both the cervical and inguinal regions through the skin. Subcutaneous tissue is dissected with a blunt haemostat taking care not to injure any vessels. Left femoral artery, left external jugular vein and right carotid artery is identified and freed from soft tissues on its anterior aspect. Insyte 24 GA catheters (Becton Dickinson Vascular Access, Sandy, UT), 0.8 mm in diameter, are used for all cannulation procedures. Catheters are made of a polyurethane elastomer approved for clinical use by the Food and Drug Administration. The catheters are inserted under direct vision into the previously dissected vessels. The left femoral artery is cannulated for measurements of MABP using a pressure transducer (Deltran, Utah Medical Products, Midvale, UT) and a recorder (Hewlett Packard 78353A). The left external jugular vein is cannulated for CVP measurements and intravenous fluid (normal saline) administration. The right carotid artery is cannulated for blood gas analysis (PaO2, PaCO2) and pH evaluation. Successful catheterization of these small vessels is only possible under direct vision because great dexterity is needed to persuade the catheter to enter the hepatic vein. Under direct vision of all the vessels, the catheter is pierced and slowly pushed up for an average of 1 cm. They are positioned carefully by fine rotational movements within the vessel until they became parallel to the cannulated vessel. Once the team is convinced about the position of the catheter, the proximal part of it fixed the superficial soft tissues in order to refrain from catheter backout. Additional parameters such as an electrocardiogram and esophageal temperature should be also measured (Fig. 14.1). The electrocardiographic probes are inserted on the distal part of the extremities, and a mini thermo probe used to monitor body temperature is located at the distal part of the esophagus through the mouth. Body temperature measurement is done with a deep esophageal thermometer (DIGI–SENSE Thermocouple Thermometer, Cole–Parmer Instrument Company, Chicago, IL) and sustained at the 36–37 with the use of an adjustable heat lamp. When all the catheter and probe placement was finished and the system was checked out for any error, the cremaster muscle is dissected on the neurovascular pedicle and prepared in the tissue bath for microcirculatory measurements.
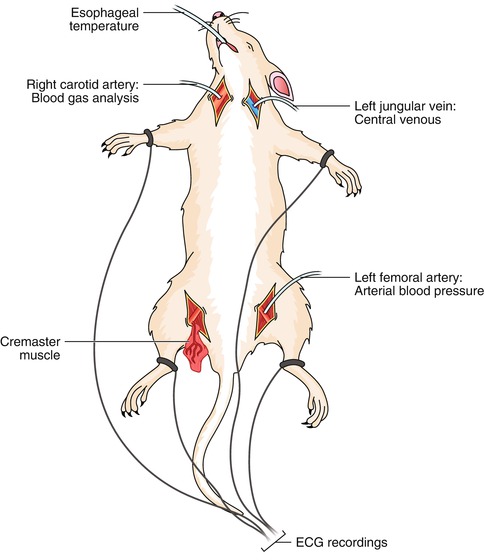

Fig. 14.1
Schematic diagram presenting different sites of cannulation for vital signs measurements
Isolation of Cremaster Muscle
Dissection of the muscle is conducted abiding to the previous description [15, 19]. An incision is made from the anterior iliac spine to the tip of the scrotum. The testis and the cremaster muscle are left intact when dissected. A cut measuring 1 cm is made on the ventral surface of the cremaster afterwards the testis and the spinal cord are removed. The neurovascular pedicle that includes the pubic epigastric arteries and the vein as well as the genitofemoral nerve is dissected with the use of a surgical microscope (Zeiss OpMi6, Carl Zeiss, Wetzlar, Germany). The neurovascular pedicle is dissected free up to the origin of this vessel from the external iliac artery and vein. Additionally, 1 cm of the nerve is excised alongside the accompanying genital branch of the genitofemoral. Finally, the front wall of the cremaster muscle sac is opened also the flap on the island cremaster muscle is created for intravital video microscopy (Fig. 14.2).
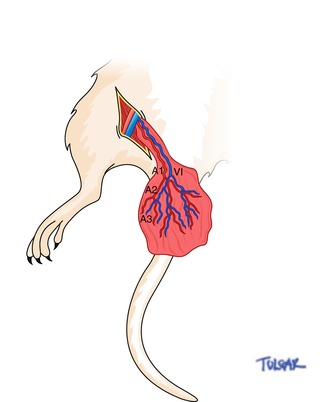

Fig. 14.2
Schematic representation of the cremaster muscle flap and vessels. A1 first-order arteriole, V1 first-order venule, A2 second-order arteriole, A3 third-order arteriole
Measurements at the Microcirculatory Level
Vessel Diameter
Video scans and diameter measurements of the segmental branch order of the cremaster arterioles and venules are taken at 1-h intervals for a 4-h period. Scans began with the major first-order arteriole (Al) and venule (VI) and progressed through the selected second-order (A2) and third-order (A3) arterioles (Fig. 14.2).
Red Blood Cell Velocities
Red blood cell velocities are recorded in the arterioles and main venule of the cremaster flap (Al, A2, A3, and VI) using a custom-made optical Doppler velocimeter from Texas A&M.
Leukocyte Count in Postcapillary Venules
Video scans of three preselected postcapillary venules (25–35/μm) are taken in proximal, middle, and distal flap regions. For each venule, the number of leukocytes rolling through the lumen, adhering to the vascular endothelium (for at least 20 s), and migrating out of the vessel wall are recorded for a 2-min period.
Functional Capillary Density
Capillary densities are measured in each of three flap regions (proximal, middle, and distal) by counting the number of flowing capillaries in the proximity to three preselected postcapillary venules. Nine visual fields of capillaries are counted at each postcapillary venule site for a total number of 27 fields per cremaster muscle flap. All scans are videotaped, allowing for repeated observations including slow-motion replay.
Discussion
The monitoring of the patients vital signs is an invaluable part of any surgical or microsurgical procedure and is considered a norm for almost all operations. Direct monitoring of vital figures includes measurements of; arterial blood pressure, central venous pressure, pulmonary arterial pressure, and others, requires introduction of arterial and venous lines by means of invasive vessel catheterization. The cremaster muscle flap model, as used before, is ideal for measurements during extensive catherization procedures. The muscle bears similarity to the skeletal muscles in the human body [20]. Its thin and translucent nature makes it ideal for vivo observation [14, 15, 21, 22], thus allowing it to be used in direct observation and quantification of microcirculatory dynamics in vivo [22].
The cremaster muscle is also a good example to the practical combination of intravital microscopy technique and microvascular research [23]. The definition of intravital microscopy can be made as the experimental approach in which the organ microcirculation is made visible under high magnification in a living animal. Advancements in the optical fields combined with the video technology has enabled us to improve imaging definition beyond 1,000-fold magnification and subsequently widened the reach of the field.
The most interesting observation in invasive catheterization is the reduction in the number of capillaries perfusing the muscle flap after the introduction of arterial and veneous catheters [24]. This finding can be correlated to the relationship that endothelial damage caused by invasive procedures, followed by vascular thrombosis and subsequent embolization from the catheter insertion site. Micro embolic and thromboembolic events are sources of severe complication following angiography, venography, lymphography, and coronarography [25–27].
As presented by oude Brink and others, thromboembolic activation preceding vessel puncture can lead to increase in emboli generation in arterioles and venues [28–31]. Our assumption is that insertion of the catheter generates superficial endothelial injury with the exposure of the subendothelial collagen. Upon this, the endothelium that has been activated has a tendency to microthrombous formation and emboli dislodgement to the peripheral tissues [24]. Blood components such as; platelets, red blood cells, and leukocytes become prone to accumulate inside and around the catheter which leads to severe microemboli formation [32, 33]. Significant documentation suggests that the passing of microemboli from the anastomotic site into the peripheral microcirculation causes vessel wall injury as well as alteration is capillary perfusion [13, 34]. Anderson and his colleagues [13] have extensively studied the effects of microemboli on peripheral microcirculatory hemodynamics. However they could not cultivate an explanation but they were able to find the factors responsible for the reduction of capillary perfusion. They developed the notion that mechanical vessel obstruction was a possible factor, and endothelium-related vasoconstriction can serve as another source of microthrombi formation [13, 34, 35]. Auckland and his colleagues [20] worked on introducing two separate risk zones into microvascular surgery. In many ways these risk zones are related to one another: a process that involves the upper affects the latter by influencing events in the downstream zone of surgical manipulation [13, 19, 34, 36]. During vessel cannulation, microthrombi and emboli are formed and then sent away from the upper zone of the catheter to the peripheral zone thus causing adverse hemodynamics effects at the micro-circulatory level [24].
A variety of mechanisms have been mentioned to explain the control of the capillary perfusion and recruitment [37]. These mechanisms include precapillary sphincters [38], functional passive sphincters within terminal arterioles [39], capillary contractility [40], pressure gradient changes [37], closure of the feeding arterioles [39, 41–43], and plugging of the capillaries by formed blood elements (WBC or RBC) [44]. We believe that the last factor suggested has a significant impact on the final outcome of microcirculatory hemodynamics [24]. But, this would affectively pose a contradiction to the role of leukocyte behavior under normal conditions [45, 46]. Their activation depends on external factors such as on tissue types, due to the capillary structure variety among different tissues. In our study we saw that direct monitoring of the cremaster flap micro vessels resulted in a significant increase in the number of activated white blood cells, both rolling and adherent to the endothelium, immediately after cannulation [24




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








