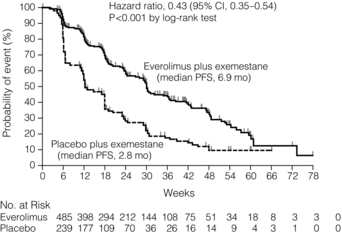
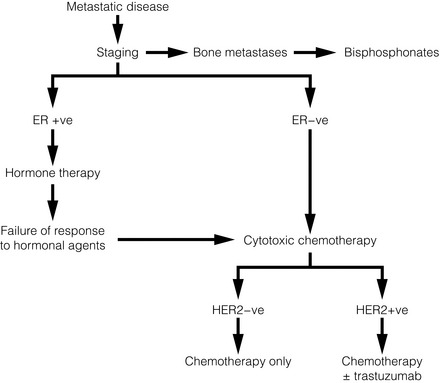
14 Metastatic spread is defined as spread of breast cancer beyond the breast and ipsilateral axillary and/or internal mammary lymph nodes. With current therapies, metastatic disease is incurable and treatment is, by definition, palliative. Such patients may, however, benefit considerably from treatment. The principles and practice of treatment include a combination of active disease management, active symptom management, and appropriate support for patient and family. A more detailed review of the evidence is provided in the National Institute of Health and Clinical Excellence (NICE) guideline.1 A minority of breast cancer patients (< 10%) present initially with metastatic disease.2 Most metastatic patients, however, present months or years after their primary treatment (surgery and appropriate adjuvant therapy). The natural history of breast cancer can be very long – patients still die from breast cancer 20 years and more after their initial treatment.3 Most patients present with symptoms of metastatic disease between follow-up visits;4 screening asymptomatic patients is not worthwhile.5,6 The common sites of metastatic spread are listed in Table 14.1; among other sites is the peritoneum, to which infiltrating lobular carcinoma, in particular, can spread and cause non-specific abdominal symptoms and/or obstruction. Table 14.1 Symptoms commonly associated with metastatic spread to different organs All patients presenting with locally advanced, inoperable or locally recurrent breast cancer should undergo a series of investigations to stage their disease adequately. In addition, patients presenting with metastatic disease at one site (e.g. bone) should have investigations to assess the extent of spread to other organs. The principal sites of spread are the thorax, bone and liver. Thus, tests to assess the extent of spread and organ function include a full blood count, clinical chemistry (urea and electrolytes, bone chemistry and liver function tests), tumour markers (carcinoembryonic antigen and carbohydrate antigen 15-3, which can be useful to assess response7), bone scintigram and computed tomography (CT) scan of thorax abdomen and pelvis. Alternatives are a liver ultrasound or magnetic resonance imaging (MRI). Increased long bone activity identified on bone scintigraphy should be further assessed by plain X-ray, supplemented by MRI if necessary, to assess degree of destruction and risk of pathological fracture. The brain should be assessed (CT or MRI) if the patient has symptoms suggestive of intracranial metastases. Urgent MRI of the whole spine is required if the patient has symptoms of spinal cord compression. Clinicians need to understand the limitations of these investigations. Although bone scintigraphy is more sensitive than plain X-ray, it will not detect all bone metastases. If a patient has persistent bony symptoms and a negative bone scan (or negative in the symptomatic area) then an MRI should be requested, since it is more sensitive than bone scintigraphy. Discrete liver metastases are well visualised by most techniques, but diffuse infiltration may not be apparent on liver ultrasound. Positron emission tomography (PET)-CT cam be useful8 in resolving whether lymph nodes or isolated lesions seen in the lung or liver are indeed metastases (albeit infected or inflammatory conditions can also be positive on fluorodeoxyglucose PET). In general, a durable response to systemic therapy offers the best quality of life (see guidance in Fig. 14.1). Figure 14.1 Outline of systemic therapy. Oestrogen receptor positive (ER + ve) includes all ER-positive and/or progesterone receptor-positive patients. ER-positive patients with lymphangitis carcinomatosa or liver metastases would normally be considered for chemotherapy in preference to hormone therapy. In 1896, Beatson10 demonstrated the endocrine sensitivity of breast cancer for the first time, by undertaking surgical oophorectomy for advanced breast cancer. Current therapy (Table 14.2) aims to either decrease levels of circulating oestrogen (ovarian ablation) or block its effect on the oestrogen receptor (anti-oestrogens). Ovarian ablation can be performed either by surgical removal (usually laparoscopically), by a short course of radiotherapy to the pelvis (infrequent – because of gastrointestinal side-effects), or the use of a luteinising hormone-releasing hormone (LH-RH) agonist, e.g. goserelin. The latter is given by monthly injection into the anterior abdominal wall and is reversible; thus, if there is no tumour response, the patient’s periods can be restored and menopausal symptoms abolished. Tamoxifen is a partial oestrogen agonist but its effects on breast cancer cells are to antagonise oestrogen. It is effective in both pre- and postmenopausal women. Table 14.2 Endocrine agents used in breast cancer Aromatase inhibitors (AIs; see next section) work in postmenopausal but not in premenopausal women. Particular caution should be taken with women who have chemotherapy-induced amenorrhoea, as they may still have some ovarian function, making AIs ineffective.12 In premenopausal women, a combination of LH-RH agonist plus AI has shown responses.13 Progestagens (e.g. megesterol acetate, medroxyprogesterone) at high dose have been used for many years, for their anti-oestrogenic action. Their main side-effects are significant weight gain and increased risk of thromboembolic disease. Suppression of glucocorticoid production has been reported,14 so patients may need hydrocortisone to cover physiological stress (e.g. pinning of pathological fractures, infections, etc.). Ovarian ablation has no role. Oestrogen in postmenopausal women is produced by conversion of androstenedione to oestrone by aromatase,15 mostly in peripheral fat but also in liver, normal breast tissue and some breast cancers. Aromatase inhibitors (Table 14.2) reduce circulating oestrogen to nearly immeasurable levels. There are two types of AI: non-steroidal (anastrozole and letrozole) and steroidal (exemestane). Their side-effects are, however, similar (Table 14.2), implying that these are due to their reduction of circulating oestrogen. Fulvestrant is a pure oestrogen antagonist, as unlike tamoxifen it has no agonist action. It binds to, blocks and degrades the oestrogen receptor. Clinical trials in postmenopausal women have shown it to be as active as anastrozole.17 It is given monthly by intramuscular injection – a potential advantage where oral compliance is a problem. Studies with higher doses, 500 mg instead of the standard 250 mg dose, have shown that these higher doses appear effective. Adding biological agents such as mTOR (mammalian target of rapamycin) inhibitors to endocrine agents such as tamoxifen or aromatase inhibitors appears to increase response rate, duration of response and even overall survival (Fig. 14.2). Studies with these agents are continuing, although as yet they are not used outside clinical trials. Chemotherapy is used to treat ER-negative breast cancer, ER-positive breast cancer that is no longer sensitive to endocrine agents, and advanced ER-positive visceral disease. The main classes of drugs and their side-effects are listed in Table 14.3. These drugs are toxic and should only be prescribed by clinicians (usually oncologists) experienced in their use. Table 14.3 Main cytotoxic chemotherapy drugs used in breast cancer *Nearly all these drugs can cause fatigue, nausea, vomiting, myelosuppression, cessation of periods (premenopausal women) and alopecia, so they are not listed individually. The drug groups with the highest activity are the anthracyclines and the taxanes. The major limitation to anthracycline use is cardiomyopathy – the risk increasing as cumulative dose increases. A course of anthracyclines cannot, therefore, usually be repeated. Taxanes are active and more effective than some other regimens.19 Capecitabine is an oral prodrug of 5-fluorouracil. It is metabolised into the active component in the liver and possibly in the tumour itself. As more agents are used in the adjuvant (or neoadjuvant) setting, the use of other active drugs such as vinorelbine, gemcitabine and platinum agents in metastatic disease is likely to increase. Platinum salts may have particular activity in the treatment of patients with basal-type tumours20 and are currently being studied in clinical trials. A growth factor receptor gene, human epidermal growth factor (HER-2), is amplified in about 14% of breast cancers and is associated with a poorer prognosis.21 Trastuzumab is a humanised monoclonal antibody that targets the HER-2 receptor in patients whose tumours overexpress HER-2 as assessed by immunohistochemistry and/or fluorescence in situ hybridisation (FISH) testing.
Metastatic disease and palliative care
Introduction
Presentation and prognosis
Site
Common symptoms
Pleura
Dyspnoea (due to effusion)
Bone
Pain
Pathological fracture
Nausea and thirst (due to associated hypercalcaemia)
Lung
Dyspnoea
Cough (dry cough is often seen with lymphangitis carcinomatosa)
Liver
Fatigue
Nausea
Anorexia
Pain over liver
Brain
Headache (often worse first thing in the morning)
Unilateral weakness
Unsteady gait
Staging
Treatment
Systemic therapy
Endocrine therapy
Class of agent
Examples
Main side-effects
Ovarian ablation
Surgical oophorectomy
Radiation menopause
LH-RH agonists
Menopausal symptoms
Anti-oestrogens
Tamoxifen
Fulvestrant
Menopausal symptoms
Thrombo-embolism
Aromatase inhibitors
Anastrozole
Letrozole
Exemestane
Menopausal symptoms
Arthralgia
Osteoporosis
Progestagens
Megesterol acetate
Weight gain
Increased appetite
Thrombo-embolism
Glucocorticoid suppression
Postmenopausal patients
Chemotherapy
Group of drugs
Examples
Main side-effects*
Anthracyclines
Doxorubicin
Epirubicin
Mouth ulcers
Cardiomyopathy
Alkylating agents
Cyclophosphamide
Antimetabolites
5-Fluorouracil
(capcitabine)
Methotrexate
Coronary spasm
Hand–foot symdrome
Taxanes
Docetaxel
Paclitaxel
Peripheral and autonomic neuropathy
Mouth ulcers
Vinca alkaloids
Vinorelbine
Peripheral neuropathy
Gemcitabine
Platinum
Carboplatinum
Cis-platinum
Neuropathy
Renal failure
Trastuzumab
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Metastatic disease and palliative care