Liposoluble
Water soluble
Others
Vitamin E (tocopherol)
Vitamin C
Idebenone
Green tea
Lycopene
Silymarin
Selenium
Curcumin
Coffea arabica and coffee berry
Resveratrol
Pomegranate
Genistein
Polypodium leucotomos
Niacinamide
Pycnogenol
The topical use of antioxidants may be effective in the prevention of skin aging. Recent surveys suggest that a combination of different antioxidants has synergistic effects and, thus, better efficacy when compared with the isolated use of an antioxidant [1, 14].
Some of the most effective:
Vitamin E: Prevents spontaneous oxidation of polyunsaturated elements and protects, in functional terms, important cellular structures, likely through inhibiting lipid peroxidation [1, 11, 12].
Coenzyme Q10 (ubiquinone): Research has shown a decline in the rate of coenzyme Q10 (CoQ10) in aged skin compared to young skin [1, 11, 12].
Idebenone (analogous synthetic of coenzyme Q10): Has been proved to be stronger than CoQ10. A study based on topical use on the skin of a compound containing idebenone has delivered positive results toward the improvement of the signs of skin aging [1, 11, 12].
Vitamin C (ascorbic acid): Plays an essential role in the synthesis of collagen and elastin, which may offset the negative effects of UV radiation on the skin. On account of its role in collagen production and the ability to eliminate damage caused by ROS, ascorbic acid has been studied for use in the treatment of the effects of aging. The dermal fibroblasts in the elderly outweigh the reduced proliferative capacity, provided treatment takes place with adequate levels of ascorbic acid [1, 11–13].
Genistein: Is an isoflavone derived from soy, possessing the ability to inhibit oxidative DNA damage caused by UVR [1, 11, 12].
32.4 Fatty Acids
With an increase in age, the characteristics of the skin change, and its capacity to combat external aggressions decreases. According to some authors, this condition is driven by changes in lipids that comprise the stratum corneum [1, 15].
Skin aging can induce epidermal lipids and the formation of free fatty acids (FFA), which, as a vicious circle, can further alter the physiological functions forming part of the skin aging process [16, 17].
A recent study analyzed the change in the composition of fatty acids in the epidermis through the process of intrinsic aging and in vivo UV exposure in human skin. The presence of 11,14,17-eicosatrienoic acid (ETA), polyunsaturated omega-3, was found to be significantly reduced in the skin, with a predominance to intrinsic aging [16, 17].
The increase in the content of ETA in the epidermis of photodamaged skin which has been acutely exposed to UV radiation is associated with the increased expression of human elongase-1 and phosphodiesterase A2, which is calcium independent. Thus, it was shown that ETA prevented the expression of MMP-1 after UV irradiation. Inhibition of the synthesis of ETA using, for example, EPTC (S-ethyl-dipropylthiocarbamate), which inhibits human elongase-1, increased the expression of MMP-1 (promoting degradation of extracellular proteins triggered by UV radiation) and contributed to the photoaging of human skin. Consequently, these results suggest that UV rays increase the levels of ETA as a photo-protective mechanism [1, 15, 16].
Fatty acids have received much attention as permeation enhancers, used to enhance the absorption of drugs and cosmeceuticals through the stratum corneum. Among the fatty acids, linoleic and oleic acids have risen to prominence [16, 17].
32.5 Anti-glycation
The understanding of the aging process involves understanding the changes that occur in molecules and their regeneration capacity. One of the biochemical reactions of this process is the nonenzymatic glycosylation, which is known to discolor and harden foods [1, 15].
Nonenzymatic glycosylation is a reaction of an aldehyde group of glucose with the amino group of a protein, to form a base (a Schiff base). This reaction usually occurs enzymatically; however, in collagen and medium- and long-living proteins, glucose can bind irreversibly without the intervention of enzymes. This spontaneous biochemical aging process contributes to the progressive damage of skin tissue and probably to the malfunction of organs (Fig. 32.1) [18, 19].
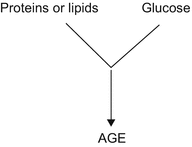

Fig. 32.1
Reaction of glycation (Maillard reaction – 1912)
The end products of advanced glycation (AGE) damage cells by way of four basic mechanisms:
Since endogenous formation of AGE is a slow process, long-lasting proteins, such as collagen, are the proteins most susceptible to the accumulation of AGE [1].
The glycation process is characterized by intra- and intermolecular cross-links, which reduce the possibility of the AGE being removed by catabolic processes, contributing to its accumulation. In collagen proteins, for example, this process contributes to the stiffness and loss of elasticity of the skin tissue. Systemic medications, researched as anti-glycation substances, are as follows: amino guanidine, acetylsalicylic acid, D3P9195, ALT 946, ALT 711, metformin, and angiotensin II receptor blockers [1, 18, 19].
Functional consequences:
Although none of medications listed above has been approved and accredited with a specific anti-AGE indication, although some anti-glycation substances are already in the preclinical and clinical testing phases [1, 18].
Most topical anti-glycation substances available on the market are intended to block the start of the glycation process, interfering in the connection between the carbonyl group of the aldehyde and the amine. The main disadvantage of this blocking process is the failure/lack of selectivity, which may cause possible interference in certain beneficial processes. Topical anti-glycation cosmeceuticals are as follows: Aldeine, Algisium C, Alistin, Ameliox, Coffee Skin, Dragosine, Trylagen, and Preventhelia [18, 19].
32.6 Cosmeceutical Metals and Ions
The use of metal ions is considered as the oldest medical text registered (about 1500 BC), namely, the Ebers papyrus of ancient Egypt. As an example, calamine (a natural material containing zinc oxide) was prescribed to treat many diseases of the skin and eyes; green minerals based on copper were used for burns and itching [1, 20, 21].
Metal ions are used as beauty products (pigments, colorants) or for skin protection (blocking ultraviolet rays). In direct contact, they not only affect the skin but may cause dermatitis by irritation or allergies if specific concentrations are exceeded [20, 22, 23].
Bioelectricity is one of the fundamental ways for the cells to communicate with each other. The skin uses these bioelectrical signals to activate the process of repair and healing. With aging, these bioelectrical signals decrease and consequently cause a reduction in the production of collagen and elastin. Research has shown that a solution of mineral ions, containing zinc and copper, combined with water can work as a “battery,” generating an electric current resulting in the inhibition of c-fos (a component of the AP-1), decreased greasiness, increased cellular adhesion, improved skin barrier structure, increased firmness, organization and repair of skin tissue, reduced skin response to stress, promotion of skin homeostasis, and inhibition of inflammation (Table 32.2) [1, 22, 23].
Table 32.2
Classification of the metals found in nature
Essential metals | Residual metals | Toxic metals |
|---|---|---|
Zinc | Zinc | Mercury |
Copper | Lead | Lead |
Magnesium | Silver | Arsenic |
Selenium | Aluminum | Aluminum |
Iron | Iron | |
Potassium | ||
Chrome | ||
Titanium | ||
Aluminum | ||
Strontium |
32.6.1 Zinc
Zinc is an essential chemical element of life. It interferes with the metabolism of proteins and nucleic acids, stimulates the activity of over 100 enzymes, contributes to the proper functioning of the immune system and wound healing, and interferes with perceptions of taste and smell, as well as DNA synthesis [22, 23].
The FDA has included zinc oxide in the list of substances generally recognized as safe for use as nutrients (GRAS). It has been proven that zinc reduces the genetic and cellular damage caused by exposure to light and enhances the strength of skin fibroblasts to oxidative stress [20–22].
Zinc oxide has been used for many years in several lip products, makeup, face powder, etc. [21, 22].
32.6.2 Copper
The number of copper compounds used in personal care products is lower. Copper peptides function to block the enzyme 5-alpha reductase, the enzyme responsible for the conversion of free testosterone into dihydrotestosterone, which in turn is responsible for the process of miniaturization of the hair follicle in androgenetic alopecia. These peptides are effective SOD-mimetic agents which catalyze the destruction reaction of superoxide anion (O2-) and, therefore, prevent this strongly degenerative free radical agent from increasing damage to the skin [1, 21, 22].
Research has also shown that its topical use, combined with vitamin C and zinc, stimulates the production of elastin [13, 20].
32.6.3 Silicon
Without the presence of silicon in the atmosphere, the existence of life in the universe would be impossible [1, 23].
Studies have demonstrated that the amount of this substance in the human body decreases with age, due to such factors as aging, exposure to ultraviolet rays, and dryness of the fabric used as a substitute for organic silicon [20, 23].
32.6.4 Magnesium
Magnesium functioning is linked to calcium. It participates in the production of specific proteins with a genetic code, contributing to the stabilization of the double helix of DNA, the synthesis and use of links with a lot of energy, as well as the synthesis and activity of multiple enzymes. In its biotechnologically bioavailable form, it energizes and tones the skin and works synergistically with zinc to promote natural revitalization. Combined with vitamin C for topical use, it functions to inhibit tyrosinase, thus promoting collagen synthesis, and also possesses anti-free radical properties [1, 20, 23].
32.6.5 Iron
Iron is a remineralizer, responsible for skin color, and an essential nutrient for oxygen metabolism and mitochondrial function. It acts and impacts on skin homeostasis and on damage repair. Iron also participates in the intracellular redox process [1, 23].
32.6.6 Selenium
The properties of capturing bioavailable selenium free radicals, biotechnologically, and its increased effectiveness make it an excellent component in formulations for skin protection (such as sunscreens and antioxidants [1, 20].
32.6.7 Aluminum
32.6.8 Titanium
32.6.9 Strontium
Its primary use when applied topically appears to be as an anti-inflammatory and anti-irritant substance [1, 22].
32.6.10 Potassium
Potassium ion is present at the ends of chromosomes (telomeres) and stabilizes the structure. The hexahydrate ion (equivalent to magnesium) stabilizes the DNA and RNA structures, offsetting the negative charge of the phosphate group [1, 20, 23].
Potassium deficiency in humans can cause acne, constipation, depression, fatigue, growth problems, insomnia, muscle weakness, nervousness, and breathing difficulty. In excess quantity, on the other hand, hyperpotassemia can cause weakness and difficulty to articulate words [21, 23].
32.6.11 Silver
Silver is toxic. However, most of the salts it contains are not absorbed and remain in the blood until deposited on the mucous membranes, forming a grayish film [1, 20, 23].
32.6.12 Lead
32.7 Moisturizers
The skin is the largest organ of the human body and provides humans with contact to the environment. Its functions are perception, thermoregulation, secretion and excretion, metabolism, and protection. It is, therefore, a complex organ that aids in the defense against the adverse effects of the external environment. To perform this task, the integument should be in its normal condition, i.e., intact [1, 24–26].
For the skin to be in its proper condition of operation, two basic processes are required: skin cleansing and moisturizing. Cleansing contributes to removing the external debris, natural skin secretions, and microorganisms. Moisturizing, in turn, guarantees the water content of the epidermis and the epidermal barrier [25, 27].
Natural moisturizing factor (NMF), intercellular lipids, and ion pumps are part of the dynamic mechanisms involved in natural hydration [1, 24].
32.7.1 Natural Moisturizing Factor (NMF) and Intercellular Lipids
The water in the epidermis is not sufficient for hydration if no similar retention factors of the same kind are present, thus preventing its evaporation. Two structures fulfill this role: the NMF and intercellular lipids [1, 27].
The keratinocytic component of the NMF has amino acids derived from filaggrin protein as the main constituent agent. The NMF retains water and ensures the normal appearance of the integument [1, 25].
The intercellular lipids, derived from nucleated keratinocytes and placed on the stratum corneum, are bipolar structures with a “hydrophilic head and hydrophobic tail.” They control the permeability and intercellular movement of water and seal the NMF in corneocytes, retaining the intercellular water content [1, 27].
The natural moisturizing factor is composed of amino acids, carboxylic acid, pyrrolidone, lactate, urea, ammonia, uric acid, glucosamine, creatinine, citrate, sodium, potassium, calcium, magnesium, phosphate, chloride, sugars, fatty acids, peptides, and other undefined substances [1, 26].
32.7.2 Ion Pumps
Next to amino acids, ionic component is the most important molecular structure of NMF, accounting for 18.5 % of this structure. These trace elements are in constant interaction with each other, balancing electrolytes. This ionic state, as well as its interface with other skin barrier structures, contributes toward establishing a suitable hydration profile [1, 28].
Ions actively participate in maintaining the water content of the intra- and extracellular environment. Of all the ion channels, the Na/K pump is best known and is responsible for maintaining the concentration of these ions [1, 28].
32.7.3 Aquaporins
Aquaporins are integral membrane proteins. There are currently 13 known species of aquaporins. Aquaporin-3 (AAQP3) stands out for being permeable to water molecules such as glycerol and urea, which are important skin moisturizing agents and known as aquaporins. On the skin, it is located in keratinocytes of the epidermis and represents a permeable channel, controlling skin hydration. The AQP3 levels may be reduced compared to high concentrations of calcium, 1.25 dihydroxyvitamin D, and UV radiation [1, 26].
The topical all-trans retinoic acid stimulates gene expression and the AQP3 protein in epidermal keratinocytes. AQP3 is also expressed in human skin fibroblasts, and normal epidermal growth factors increase its expression and cell migration [24, 26].
The relevance of AQP3 in skin diseases associated with abnormalities in water homeostasis, such as atopic dermatitis, psoriasis, xeroderma, and ichthyosis, still needs to be established and so too its beneficial potential to modulate the function of AQP3 with topical inhibitors or activators. Aquaporin research in the field of skin hydration, however, is pointing toward potential interesting benefits in addressing primary xerosis [24, 26].
32.7.4 Classification of Moisturizers
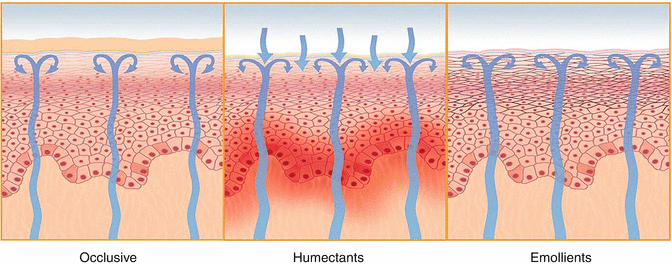
Moisturizers are classified according to the action mechanism of their compounds. Thus, they can be classified as occlusive, emollients, and humectants (Table 32.3 and Fig. 32.2) [1, 27].
Table 32.3
Examples of moisturizers
Occlusive | Humectants | Emollients |
|---|---|---|
Hydrocarbon oils/waxes | Glycerin | Protective emollients |
Petrolatum | Honey | Diisopropyl dimer dilinoleate |
Mineral oil | Ammonium lactate | Isopropyl isostearate |
Paraffin | Urea | Castor beans |
Scalene | Propylene | Fat liquors |
Silicone derivatives | Sodium pyrrolidone carboxylate (sodium PCA) | Propylene |
Dimethicone | Hyaluronic acid | Jojoba oil |
Cyclomethicone | Sorbitol (glucitol) | Ceramides |
Phospholipids | Panthenol | Octyl octanoate |
Fatty alcohols | Polyglycerylmethacrylate | Isopropyl palmitate |
Sterile alcohol | Gelatin | Glycol stearate |
Lanolin alcohol | Sodium lactate | Lanolin |
Lecithin | Cetyl stearate | |
Sterols | Hexyl dodecanol | |
Candelia | Oleyl alcohol | |
Lanolin acid | Soybean sterols | |
Cholesterol | ||
Vegetable waxes | ||
Beeswax |
Most frequently, commercially available products use compounds of each of these classes in their formulations. The composition of a moisturizer is the secret to its success [27, 28].
32.7.4.1 Occlusives
These are products rich in occlusive components which slow the evaporation and epidermal loss of water through the formation of a hydrophobic film on the skin surface and interstitium, between the surface keratinocytes. These are usually fatty compounds, more effective when applied to the slightly moist skin. Although greasy, they may present an oil-free profile [1, 27, 28].
32.7.4.2 Humectants
These are products comprised of substances that retain water in the horny outer layer, or draw water from the dermis, or, alternatively, in environments with atmospheric humidity greater than 70 %, draw water from these environments. These compounds are associated with occlusive compounds. The cosmetic actions of humectants are directly proportional to the concentration used, as well as its adverse effects [1, 27, 28].
32.7.4.3 Emollients
Known as products with “special mechanisms,” emollients are rich in compounds capable of filling the inter-corneocytic cracks, retaining water in this layer. Such hydrating capacity is achieved by increasing the cohesion between these cells, increasing the natural “occlusive” capacity of the horny layer of the skin [1, 27, 28].
32.7.5 New Categories of Moisturizers
Some authors establish two new classes of commercial skin moisturizers, namely, protein repairers (e.g., collagen) and barrier restorers (e.g., N-palmitoyl ethanolamine, ceramides, polyunsaturated fatty acids, omega-3 complex, and liposomes). The former are classified as occlusive agents, and the barrier restorers are considered as emollients [1, 27, 28].
32.8 Microdermabrasion
Microdermabrasion is an important process in skin rejuvenation, as it accelerates the process of tissue repair, increasing desquamation of epidermal cells, therefore bringing about cell renewal and elimination of dead skin cells. The reduction of cell cohesion promotes skin softness and facilitates the penetration of antiaging products [1, 25, 29].
Microabrasive agents promote exfoliation, whether physical or chemical, and cell renewal. Silica, microspheres of jojoba, walnut shell powder, and Fiber T1 are physical exfoliants that promote a mechanical exfoliation, facilitating the loss of cell adhesion in the surface stratum corneum. On the other hand, chemical exfoliants decrease the cohesion between the corneocytes by different mechanisms. Good examples are retinoids and hydroxy acids [1, 9, 29].
In addition to these active agents, the pharmaceutical and cosmetic industries have provided new direct microabrasive agents (whose main action is to promote desquamation) and indirect microabrasive agents (which operate in basal keratinocytes or in fibroblasts, thus increasing cell renewal and, secondarily, skin peeling) [1, 29].
32.8.1 Microabrasive Cosmeceuticals with Direct Action
32.8.1.1 Physical
Farmal (Fiber T1)
A compound composed of tapioca fiber extracted by means of physical separation, solvent-free, in a process that preserves within its composition a portion of starch which confers on the skin proper exfoliation with an ultimate sensation of softness. It is recommended for stimulating skin cell renewal following the physical process of exfoliation [1, 29].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree