The incidence of cutaneous malignant melanoma continues to increase worldwide. It is the deadliest form of skin malignancy. This article focuses on the epidemiology, diagnosis, prevention, and new staging criteria of melanoma. The author delves into standards of treatment and highlights new strategies, including but not limited to noninvasive imaging techniques and immunotherapy.
Key points
- •
In the United States, the incidence of melanoma is increasing with an estimated 76,250 cases in 2012.
- •
New 2009 AJCC staging criteria allows for increased accuracy of diagnosis and treatment.
- •
The new adjuvant therapies, ipilimumab and vemurafenib, offer promising options for therapy.
- •
Melafind, a new innovation computer-based visual technology, may improve the accuracy in diagnosis of cutaneous melanoma.
Introduction
In simple terms, melanoma is described as malignancy of the melanocyte, a melanin-producing cell located in the stratum basale of the epidermis. Normal-functioning melanocytes remain responsible for the basic pigmentation of the skin, in addition to protection versus harmful UV light. Practitioners commonly refer to this malignancy as either cutaneous or malignant melanoma.
Introduction
In simple terms, melanoma is described as malignancy of the melanocyte, a melanin-producing cell located in the stratum basale of the epidermis. Normal-functioning melanocytes remain responsible for the basic pigmentation of the skin, in addition to protection versus harmful UV light. Practitioners commonly refer to this malignancy as either cutaneous or malignant melanoma.
Epidemiology
Worldwide, the incidence of cutaneous melanoma is increasing continuously, especially among whites. Estimated cases in the United States in 2012 were 76,250 new diagnoses with concurrent 9180 deaths expected because of melanoma alone. Surveillance Epidemiology and End Results data collected from the National Cancer Institute also conclude that this cancer is steadily increasing among whites with a 60% increase observed within the last 30 years. These data quote melanoma as the fifth and seventh most common new diagnosis of malignancy among men and women, respectively.
Incidence rates of melanoma per year vary between women and men from 3% to 4.1% per year and 5.1% to 6.1% per year, respectively. These rates have been reported as high as 21.6, 1, and 4.5 per 100,000 among non-Hispanics, African Americans, and Hispanics, respectively. Overall incidence seems to be higher among females; the elderly; and non-Hispanic whites versus Hispanic whites, African Americans, American Indians, Alaskans, Asians, and Pacific Islanders. However, minority groups demonstrate increased metastasis, advanced stages, thicker lesions, earlier age at diagnosis, and poorer outcomes overall.
Anatomic location of these lesions also helps study the evolution of melanoma throughout the last several decades. During the 1970s, cutaneous melanoma was mainly observed on the trunk of males and extremities of females. However, most likely because of changes in lifestyle habits and clothing, current reports state lesions are most common on the chests of females and lower extremities of males. Different patterns of sun exposure help predict location of such lesions. Head, neck, and extremity lesions are associated with chronic sun exposure versus truncal lesions associated with intermittent UV radiation.
Many suggest that there is currently no decline in the mortality rate of this disease. Median survival rates after metastasis occurs have been reported as a mere average of 6 to 9 months, with records of a 5-year survival rate less than 5% to 10%.
Risk factors
Several risk factors are linked to the development of cutaneous melanoma. Genetic predisposition plays a large role and several susceptibility genes have been identified. An important gene, CDKN2A, codes for a tumor-suppressor protein, p16INK4A. This is a CDK4 inhibitor causing the phosphorylation of Rb, which regularly restricts the G1-S interface during cell division in mitosis. This mutation, witnessed in a third of the cases of hereditary melanoma, thus disrupts the normal cell division pathway.
Another significant gene, BRAF (V600E), is thought to be crucial to the pathogenesis of melanoma. Several studies implicate that a single mutation in this gene is observed in 50% to 60% of cutaneous melanomas. These mutations are often associated with younger patients (86% between 20 and 30 years old); a predilection for the trunk and extremities; high nevus counts; few freckles; individuals that tan easily; and the superficial spreading subtype. Other susceptibility genes include NRAS (15%–20%); CKIT (2%, 10%–20% acral or mucosal melanomas); GNAQ/GNA11 (50% uveal cases); BRCA2; MCR1; OCA1; and those with an inherited defect causing xeroderma pigmentosum.
Studies report that 1% to 11% of patients with prior history of melanoma develop additional primary melanomas. Additionally, family history cannot be ignored, especially with the implication of genetic susceptibility. As many as 8% to 12% of patients with melanoma showed familial propensity in certain studies, with an individual with one first-degree showing a 1.7-fold increased risk, whereas a ninefold increase was appreciated in those patients with two affected first-degree relatives.
Age plays a large role in melanoma with the median age of diagnosis at 62 and 54 for men and women, respectively. In addition to standard screening used for skin cancer, men older than age 50 should be specifically targeted because of this increased propensity. Other risk factors include moles that are new, changing, or symptomatic, and patients with prior removal of suspicious moles. Chronic immunosuppression also seems to play a large role, especially in those patients with prior cancer (chronic lymphocytic leukemia and non-Hodgkin’s lymphoma especially), AIDS, or posttransplant. Melanoma was observed in 6% to 15% of posttransplant cases of skin cancers.
In summary, the strongest causes of melanoma can be linked to a personal or family history of melanoma, male gender, older age, presence of dysplastic or atypical nevi, history of intense intermittent sun exposure, and increased sunburns during childhood. Researchers have found that the HARMM acronym can help identify the five most important independent factors for increased likelihood of melanoma. A score of five represents high-risk patients, whereas 0 to 1 confers lower risk. This simplifies the importance of history of prior melanoma (H), age greater than 50 years (A), the absence of a regular dermatologist (R), a changing mole (M), and the male gender (M).
Furthermore, environmental exposures play an important role in melanoma. Researchers have concluded that consistent residence in medium or high UVR locations is associated with an incremental risk of two Non-Melanoma Skin Cancer or greater later in life. Concomitantly, sun exposure is the most important environmental risk and the only one that remains potentially modifiable. Sun exposure and its’ association with melanoma seems to increasingly affect those individuals who experience a pattern of intense intermittent exposure versus chronic exposure, thus concluding that vacationing and recreational activities are modifiable risk factors.
Artificial UV exposure by tanning is an additional modifiable risk factor. This source of intermittent radiation became popular in the 1980s and emits UVA and UVB radiation. UVB rays are the putative factor in the carcinogenesis of skin cancer; however, individuals must remember that UVA can also be absorbed by melanocytes and is much higher in artificial sources than in natural exposure. A study in Minnesota observed that a strong dose response relationship between the risks of malignant melanoma existed between the total hours, sessions, or total years that individuals spent with indoor tanning. Cutaneous malignant melanoma was observed higher among indoor tanners versus those who never used it (odds ratio, 1.74). An additional analysis of artificial sources concluded that an increased risk (relative risk, 1.15) existed for any individual that had ever used a sunbed, and even higher was observed (relative risk, 1.75) if this occurred before the age of 35. These studies concluded that no artificial device is safe, regardless of the UVA/UBV emission ratio.
A clear link exists between atypical or dysplastic nevi and malignant melanoma. Typical features of dysplastic nevi include lesions that are greater than 6 mm in diameter, are flat, possess irregular borders, and contain a variability in color. However, dysplastic nevi individually rarely evolve into melanoma. Although a single dysplastic nevus does not hold much malignant potential, multiple nevi are markers for malignancy. Congenital nevi, melanocytic nevi that are present at birth, also confer risk for future melanocytic lesions. These are sometimes evident in 1% to 6% of neonates and risk is dependent on size. A small (<1.5 cm) or medium lesion has a low (<1%) lifetime and nonexistent risk before puberty. However, a larger lesion (>20 cm) contains a 5% risk of evolving into either a cutaneous or extracutaneous melanoma.
Diagnosis
For a clinician to properly identify and manage cutaneous melanoma, it is imperative to be aware of the array of lesions encompassing the differential diagnosis for melanoma. These lesions include seborrheic keratosis, pigmented actinic keratosis, pigmented basal cell carcinoma, blue nevi, and benign nevi.
After proper identification of a lesion that is suspected to be melanoma, the clinician must do a proper clinical examination to determine if it is consistent with melanoma. This must include a total skin examination from head to toe with emphasis placed on sun-exposed areas including the scalp, face, extremities, and back. Caution must be taken to include intertriginous regions, palms, soles, and the nail beds because in situ lesions may show up here. Afterward, the five critical fundamentals of determination can be explored: (1) asymmetry, (2) border, (3) color, (4) diameter, and (5) elevation.
Important parameters to consider include examining the pattern and the shape of the lesion. Irregular borders of melanoma are often attributed to the propensity of the lesion for uneven growth within it. The most important determination of color includes irregularity and specific colors. Cutaneous melanomas are often blue-black; however, numerous colors, such as brown, tan, red, gray, or white, can be demonstrated within these lesions. A specific shade of gray or white often represents an area of regression within the lesion, one where melanin production has concurrently stopped and left behind a lesion resembling a pale scar. Color can also help determine subtypes of melanoma. Uniformly black lesions are often small and nodular in type.
An important method to aid in the diagnosis of melanotic lesions includes dermoscopy. A dermatoscope allows for the magnification of lesions while simultaneously providing a polarized light source rendering the stratum cornea translucent. Two key objectives of dermoscopy are decreasing the number of biopsies of benign skin lesions in addition to providing increased diagnostic accuracy of melanoma. Several dermatoscopic structures associated with an atypical proliferation include an atypical pigment network, pigment pseudonetwork, irregular dots and globules, streaks, and even a blue-white veil overlying the suspected lesion. An atypical lesion has grid lines of varying thickness and color, whereas a benign lesion demonstrates a regular meshlike appearance.
Classification
Melanoma is classified as superficial spreading, nodular, lentigo maligna, acral lentiginous, and desmoplastic melanoma. Further subtyping in the future could result in a more refined classification system possibly personalizing specific treatments.
Superficial spreading melanoma is often seen in younger patients with a median age in the fifth decade ( Fig. 1 ). Lesions present as flat, slow-growing, irregularly bordered lesions with variegated pigment. These lesions enlarge radially and are present on areas of intermittent sun exposure including the trunk, back, and extremities. Histology demonstrates large pleomorphic epitheloid melanocytes in nested and single cells, upward migration demonstrating pagetoid epidermal invasion ( Fig. 2 ). Nodular melanomas can present on any location as rapidly expanding nodules, which may ulcerate and hemorrhage ( Fig. 3 ). Epidemiologically, this is often seen in older patients near the seventh decade of life. Histology demonstrates no epidermal portion of the lesion extending beyond the dermal component ( Fig. 4 ).
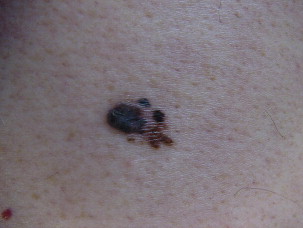
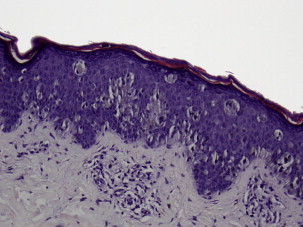
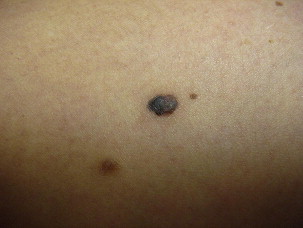
An additional subtype found in the elderly includes lentigo maligna, mainly observed near the eight decade ( Fig. 5 ). These lesions are typically found on chronically sun-exposed areas including the head, neck, and forearms. Lesions demonstrate large variegated pigmented macules with irregular edges, which can further become raised if dermal invasion occurs. Histologically, severe dermal solar elastosis and epidermal thinning with confluent melanocytes, and pagetoid epidermal invasion in the epidermis are characteristic ( Fig. 6 ). Acral lentiginous melanoma is found specifically on the palm, soles, or subungal locations ( Fig. 7 ). These are very slow growing and demonstrate variegated pigmented macules. Desmoplastic melanoma demonstrates more sarcoma-like tendencies with increased hematogenous spread when compared with other subtypes. Postoperative radiotherapy must be offered to these patients because of the increased local recurrence rates even with adequate excision margins.
Staging
New staging criteria by the American Joint Committee on Cancer published in 2009 allow for increased accuracy and precision for clinicians managing malignant melanoma compared with the 2002 guidelines ( Tables 1 and 2 ). The American Joint Committee on Cancer 2009 guidelines reiterated the importance of several prognostic factors including tumor thickness and mitotic rate, and significantly the mitotic rate now replaces previous Clark’s level of invasion for criteria in defining stage T1b melanomas. Any patient with microscopic nodal metastases is now officially classified as stage III.
| Classification | Thickness (mm) | Ulceration Status/Mitoses |
|---|---|---|
| T | ||
| Tis | NA | NA |
| T1 | ≤1.00 | a: Without ulceration and mitosis <1/mm 2 b: With ulceration or mitoses ≥1/mm 2 |
| T2 | 1.01–2.00 | a: Without ulceration b: With ulceration |
| T3 | 2.01–4.00 | a: Without ulceration b: With ulceration |
| T4 | >4.00 | a: Without ulceration b: With ulceration |
| N | No. of Metastatic Nodes | Nodal Metastatic Burden |
|---|---|---|
| N0 | 0 | NA |
| N1 | 1 | a: Micrometastasis a b: Macrometastasis b |
| N2 | 2–3 | a: Micrometastasis a b: Macrometastasis b c: In transit metastases/satellites without metastatic nodes |
| N3 | 4+ metastatic nodes, or matted nodes, or in transit metastases/satellites with metastatic nodes |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





