Mandible Reconstruction
Joseph J. Disa
Evan Matros
The mandible contributes to airway stability, is important in speech, deglutition, and mastication, and largely determines the shape of the lower face. Consequently, functional and aesthetic goals are equally important considerations in mandible reconstruction. Specific functional goals include preservation of temporomandibular joint function with maximal opening ability and maintenance of occlusion. In more severe cases in which many teeth are missing, restoration of normal interarch distance and alignment is critical for the facilitation of subsequent dental rehabilitation. Key aesthetic goals include symmetry, preservation of lower facial height and anterior chin projection, and correction of submandibular soft-tissue neck defects.
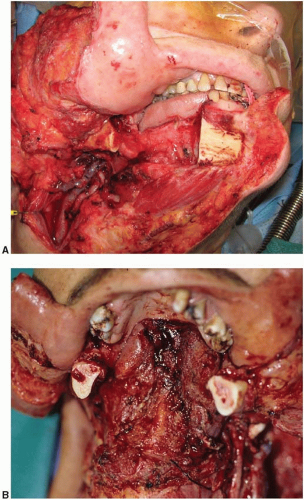
The vast majority of segmental mandible defects are caused by cancer. Squamous cell (epidermoid) carcinoma is the etiology in the majority of cases with the mandible commonly invaded by adjacent tongue or floor of mouth tumors. Osteogenic sarcoma is the second most common cause of segmental mandibular defects resulting from cancer resection and the most common primary bone tumor. Mucoepidermoid carcinoma, adenoid cystic carcinoma, leiomyosarcoma, and fibrous histiocytoma are examples of other tumors. A small number of segmental mandibular defects result from extensive benign cystic or fibrotic bone disease. Gunshot wounds are the most common traumatic cause, but their number is small compared with tumors. Segmental loss because of infection is rare, but can occur after complications of mandible fractures.
Mandible defects requiring reconstruction are sometimes caused by bone loss alone (e.g., osteoradionecrosis). However, the majority of defects usually include adjacent intraoral soft tissue as well as submandibular soft tissue. Some bone defects include external skin loss instead of mucosa, and the most complex include bone, mucosa, and skin.
Two classification schemes have been proposed for mandible defects. The most practical describes bone loss in terms of central segments (designated C and defined as lying between the two canine teeth), lateral segments (L), and hemimandible segments (H).1 Hemimandible and lateral segments are similar except that the former includes the condyle, whereas lateral segments do not. A defect commonly is a combination of more than one segment, for example, LC, HC, or LCL. Although this description may appear tedious, it is actually useful as a common language to standardize the variable reconstructive problems posed by these entities (Figure 37.1).
Mandible reconstruction can be accomplished by a variety of means, including nonvascularized bone grafts, metal plates, pedicled flaps, and free flaps. Nonvascularized grafts, such as an iliac crest segment, can be used for a short bone gap (<3 cm) in a setting of benign disease. This is a rare application. Although conceptually and technically simple, this method relies on creeping substitution for long-term mandible stability.
Pedicled flaps include the trapezius and pectoralis osteomyocutaneous flaps. The primary attraction of these donor sites is that they lie adjacent to the head, thus permitting their movement into this area without disconnecting their blood supply. Although this is an attractive concept, there are several important drawbacks. First, use of these flaps enlarges the size of the primary wound considerably compared with harvesting tissue from a distant donor site. This increases the potential for morbidity at the site of the reconstruction. More importantly, a significant portion of flap volume is used just to reach the recipient site. The distal portion of the flap, which is used for the actual reconstruction, often has a marginal blood supply and is at risk for ischemic necrosis. Perhaps the greatest limitation of these flaps is that they do not provide enough tissue in the proper configuration to be useful. The bone available with the pectoralis major muscle (rib) and the trapezius (spine of the scapula) is limited compared with free-flap alternatives. In addition, the osseous components of these flaps have poor blood supply, derived only from the periosteum, resulting in high rates of non-union and limiting the surgeon’s ability to perform shaping osteotomies. Although the pectoralis has been used to reconstruct the anterior mandible and the trapezius to reconstruct the lateral mandible, these flaps are generally not recommended as primary methods of mandible reconstruction.
Prosthetic mandible replacement has evolved as an alternative method of reconstruction that still has legitimate, but limited, application. Mesh trays made of Dacron or metal were introduced in the 1970s as scaffolds that were filled with bone graft chips and used for segmental bone defects. Long-term follow-up has shown this method to be ineffective. Problems with extrusion and bone graft dissolution commonly occurred. Metal reconstruction plates developed as a result of orthopedic hardware advances in other areas. These plates are available today in a variety of lengths and styles.
Metal reconstruction plates offer advantages of decreased operating time and avoidance of a bone graft donor site. They have important disadvantages: risk of exposure or infection; risk of plate fracture; preclusion of dental reconstruction; and a thin shape that does not provide adequate bulk for reconstruction. These disadvantages are particularly problematic in the setting of radiation therapy. Another important drawback is the functional limitation seen with the use of metal plates for hemimandible defects that include the condyle. The prosthetic condyle is a poor substitute for the native structure. The long-term effects of a metal condyle in the native glenoid fossa are unknown, and occlusion is often poorly maintained with a metal plate that includes a condyle. As a result of these disadvantages, the first choice for reconstruction of segmental mandibular defects is with vascularized bone flaps. However, prosthetic reconstruction may be useful in scenarios when bone reconstruction is not possible such as extensive oncologic resection, absence of suitable bone flaps, or presence of significant medical comorbidities.
When reconstruction of segmental mandibular defects is performed with reconstruction plates, adequate soft-tissue coverage is critical to prevent plate extrusion. The pectoralis major myocutaneous flap is commonly used for this purpose; however, plate exposure still occurs, particularly with anterior reconstructions in which the tension on the flap is greatest. One in three plate reconstructions fails when a pedicled flap is used for coverage.
The most reliable soft-tissue coverage for a reconstruction plate is provided by a free flap, which provides abundant tissue and can be inset without tension. The vertical rectus flap, forearm flap, or the anterior lateral thigh (ALT) flaps are commonly used for this purpose and flap selection is guided by the volume of soft tissues required for reconstruction. The sole advantage of this approach (reconstruction plate plus forearm flap) is that it is somewhat quicker to perform than
an osteocutaneous free flap. The disadvantage is that it combines the worst features of its two component parts: the risks of infection and exposure from a foreign body and the risk of free-flap failure. The combination of a soft-tissue free flap and a reconstruction plate is probably best reserved for lateral defects in those patients who are poor candidates for an osteocutaneous free flap. Elderly patients with multiple medical comorbidities may benefit from a shorter operative procedure and are more likely to accept the permanent dentition defect than are young patients.2
an osteocutaneous free flap. The disadvantage is that it combines the worst features of its two component parts: the risks of infection and exposure from a foreign body and the risk of free-flap failure. The combination of a soft-tissue free flap and a reconstruction plate is probably best reserved for lateral defects in those patients who are poor candidates for an osteocutaneous free flap. Elderly patients with multiple medical comorbidities may benefit from a shorter operative procedure and are more likely to accept the permanent dentition defect than are young patients.2
Osteocutaneous free-flap reconstruction is often the most effective method of mandible repair. These flaps have both soft tissue and bone components, which are available in an optimal configuration for solving specific composite tissue problems. This technique is ultimately dependent on the integrity of the microvascular anastomoses for success. Fortunately, the favored donor sites all have excellent flap pedicle qualities (vessel diameter and length), and the head and neck area generally has excellent recipient vessels available, despite previous surgical treatment and radiation. Free-flap survival rates are approximately 95%.3
FREE-FLAP DONOR-SITE SELECTION
Since early in the development of free-flap mandible reconstruction, there have been multiple donor sites from which to choose. Rib, metatarsal, and ilium were among the first flaps developed.4 The ilium had been the most popular of the three owing to its abundant bone, which even resembles a hemimandible when harvested in a particular way and was the workhorse for the first decade of free-flap mandible reconstruction. Further evolution has led to the development of the radius, scapula, and fibula donor sites.5 These additional options have increased the flexibility and precision of the technique as the specific assets and limitations of each donor site have become clear.
A review of 155 free-flap mandible reconstructions at Memorial Sloan-Kettering Cancer Center has shown that the fibula is currently the donor site of choice for most patients (Table 37.1).6 The radius, the scapula, and the ilium (to a diminishing extent) are better choices in a few specific settings. Each has unique advantages and disadvantages. A comparison of the donor sites is helpful in selecting the proper flap for a particular problem (Table 37.2). Some have better bone qualities, some have better skin, and some have significant disadvantages that make them seldom the flap of choice despite their good qualities (Figure 37.2).
Ilium
The ilium has abundant bone but has a predetermined shape that makes flap shaping inherently less precise than other donor-site options. It may be useful in some hemimandible reconstructions because its shape most closely resembles this portion of the mandible. The ilium is said to have a segmental blood supply from the deep circumflex iliac artery, although this is debatable on a practical level. This type of vascular anatomy is preferred because it allows segmental osteotomies with survival of each portion of the flap. Long ilium flaps, however, tend to have less robust, even marginal, circulation at the distal end of a multiply osteotomized flap.
The skin island available with the ilium does not have a reliable circulation in many patients. In addition, the soft-tissue component of the flap is often bulky and lacks mobility with respect to the bone. This makes insetting difficult and limits usefulness of the soft-tissue component of the flap. Some authors propose including a portion of the internal oblique muscle with the flap as an alternative source of soft tissue. The muscle is covered with a skin graft when used inside the oral cavity.
Closure of the ilium donor site is arduous and there is a possibility of hernia formation or late attenuation of the lateral abdominal wall. This donor site is painful and limits early mobilization of the patient. Splitting the ilium and leaving its outer rim intact is proposed as a means of facilitating the closure process, but this makes flap harvest more tedious.
TABLE 37.1 FREE-FLAP DONOR-SITE SELECTION IN MANDIBLE RECONSTRUCTIONa | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||
TABLE 37.2 FREE-FLAP DONOR-SITE COMPARISON FOR MANDIBLE RECONSTRUCTIONa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree