17 Management of Dupuytren’s disease
Synopsis
 Dupuytren’s disease is a benign fibromatosis of the palmar and digital fascia characterized by nodular thickening and subsequent contracture.
Dupuytren’s disease is a benign fibromatosis of the palmar and digital fascia characterized by nodular thickening and subsequent contracture.
 Successful treatment requires a detailed understanding of the normal palmar and digital fascial structures and their corollary diseased states.
Successful treatment requires a detailed understanding of the normal palmar and digital fascial structures and their corollary diseased states.
 Operative indications are based on the degree of contracture and the resultant functional disability of the hand.
Operative indications are based on the degree of contracture and the resultant functional disability of the hand.
 Palmar fasciectomy remains the mainstay of surgical treatment.
Palmar fasciectomy remains the mainstay of surgical treatment.
 Evolving treatments include percutaneous (needle) aponeurotomy and collagenase injection.
Evolving treatments include percutaneous (needle) aponeurotomy and collagenase injection.
 Despite appropriate management, recurrence and disease progression remain the primary obstacles to treatment.
Despite appropriate management, recurrence and disease progression remain the primary obstacles to treatment.
Introduction
Epidemiology
Dupuytren’s disease belongs to the overarching category of benign superficial fibromatoses and, as such, is closely related to Peyronie’s disease (penile fibromatosis) and Ledderhose’s disease (plantar fibromatosis). Historically, Dupuytren’s disease has been successively referred to as a Nordic, Caucasian, Anglo-Saxon, and finally a Viking disease.1–4 Although most prevalent in populations with a strong northern European ancestry, Dupuytren’s disease affects populations throughout the world. Critical evaluations of the migrations that have occurred throughout history, particularly those originating in northern Europe, suggest that the disease likely originated among Celtic and Germanic tribes around 1200 bc and dispersed throughout Europe with the great migrations beginning around 200 bc.5 Few populations are spared at least anecdotal cases of Dupuytren’s disease; however, the incidence remains highest among individuals of northern European ancestry, with overall estimates ranging from 2% to 42% depending on the population being studied.6 Men are six times more likely to develop Dupuytren’s disease than are women and typically exhibit an onset in the fifth decade of life, in comparison to onset within the sixth decade for women.7 Population studies support an autosomal-dominant inheritance pattern with variable penetrance.6
Associations with repetitive trauma, alcohol abuse, hepatic disease, diabetes mellitus, smoking, chronic obstructive pulmonary disease, human immunodeficiency virus, malignancy (paraneoplastic manifestation), and epilepsy have been proposed.8–10 These associations are often weak and most likely represent factors that may increase the penetrance or exacerbate an underlying genetic predisposition.
Palmar and digital fascia
Palmar fascia
The palmar fascia provides a flexible yet firm framework for the soft tissues of the palm, tethering the skin to the underlying musculoskeletal structures. Classic descriptions of the anatomy of the palmar fascia provided by Legueu, Juvara, and Testut have withstood the test of time.11,12 The palmar fascia consists of two distinct layers: the deep fascia and the superficial fascia or palmar aponeurosis. The deep fascia covers the interosseous muscles and is not involved in Dupuytren’s disease. The superficial fascia, in contrast, is affected by the pathologic progression of Dupuytren’s disease.
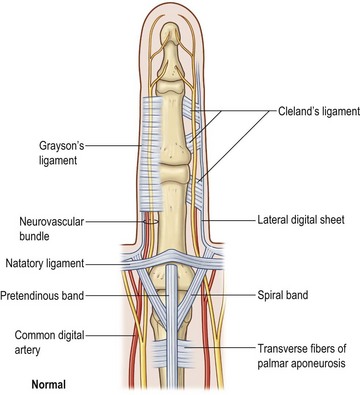
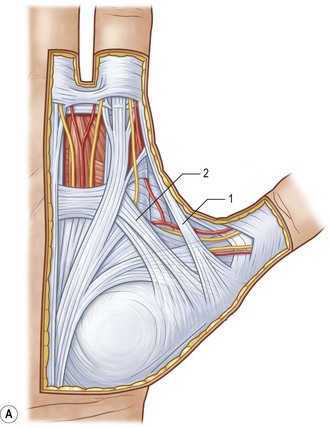
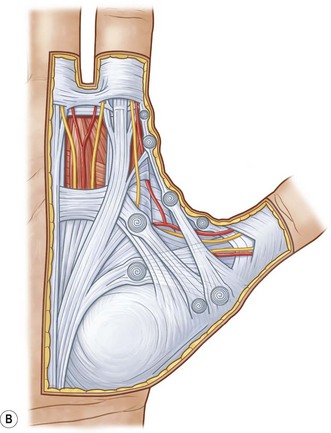
The palmar aponeurosis is a triangular-shaped fascial structure consisting of longitudinal, transverse and vertical fibers with its apex in continuity with the palmaris longus tendon (Fig. 17.1). Although these structures are in continuity, the palmar aponeurosis is histologically distinct from the palmaris longus and is invariably present even in patients with an absent palmaris.
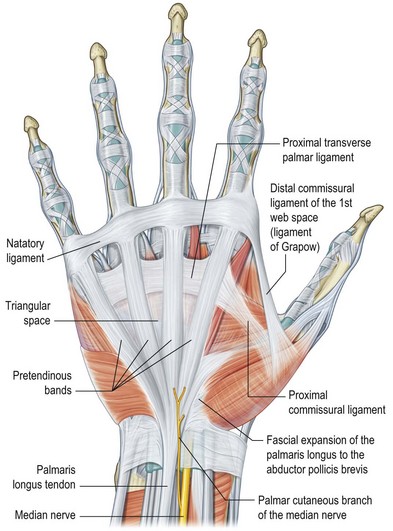
Fig. 17.1 Anatomy of the palmar fascia.
(Redrawn from Tubiana R, Leclercq C, Hurst L, et al. (eds) Dupuytren’s Disease. London: Martin Dunitz, 2000, p. 22.)
The longitudinal fibers course superficial to the flexor retinaculum, forming pretendinous bands. These bands travel distally and insert into the deep surface of the dermis at the distal palmar crease, contribute to the retrovascular fascial structures, and bifurcate around the flexor tendon sheath to insert on the radial and ulnar sides of the MCP joint.13
The transverse fibers are characterized by two distinct bands, one proximal and one distal. The proximal transverse fibers, located at the level of the distal palmar crease, course deep to the longitudinal pretendinous bands and are not typically affected by Dupuytren’s disease.14 Radially, these fibers form the proximal commissural ligament of the first webspace. The distal transverse fibers, alternately referred to as the natatory ligament, course superficial to the longitudinal pretendinous bands and are affected by Dupuytren’s disease. The natatory ligament extends from the radial border of the index finger to the ulnar border of the small finger. Ulnarly, the natatory ligament divides to envelop the abductor digiti minimi (ADM) and the ulnar neurovascular bundle. Radially, the natatory ligament is continuous with the distal commissural ligament of Grapow within the first webspace.
The vertical fibers of Legueu and Juvara connect the superficial palmar aponeurosis to the deep fascia. These fibers form a series of eight vertical septa on the radial and ulnar sides of the flexor digital apparatus. These septa divide longitudinal compartments containing the flexor tendons from those containing the lumbricals and digital neurovascular bundles.11,15 Additional vertical fibers connect the superficial palmar fascia to the overlying dermis, providing resistance to shear forces within the palm.13
Digital fascia
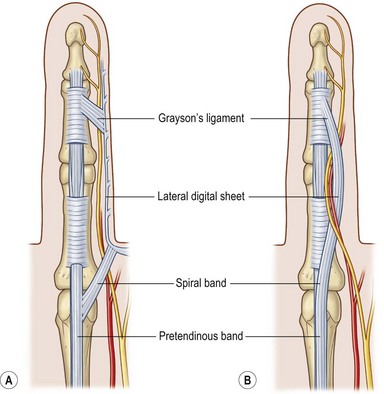
The palmar and digital fascia is contiguous with a complex digital–palmar junction in which intermediate and deep fibers of the longitudinal bands bifurcate, contributing to the formation of digital retrovascular bands. Anatomic studies have revealed more variability in the anatomy of the digital fascia in comparison with the relatively constant palmar fascial anatomy; however, relative agreement exists over the basic structure. The finger is invested by an elliptical fascial covering consisting of volar and dorsal fascial sheets that lie superficial to the flexor and extensor mechanisms respectively. These sheets unite along the radial and ulnar aspects of the finger. The volar and dorsal aspects of the finger are separated by a series of lateral structures that envelope the neurovascular structures of the finger.16 These lateral structures include Cleland’s ligaments, Grayson’s ligaments, and transverse retinacular ligaments. Cleland’s ligaments consist of a series of dorsal fiber bundles that arise proximal and distal to the interphalangeal joint, fanning laterally to insert in the skin. These fiber bundles do not form a contiguous sheet and run within several planes dorsal to the neurovascular bundle. Grayson’s ligaments are more distinct, arising from the volar surface of the flexor tendon sheath, fanning laterally to insert into the skin, volar to the neurovascular bundle. The transverse retinacular ligaments originate from the volar capsule of the PIP joint and course dorsally to insert into the lateral margin of the extensor mechanism17 (Fig. 17.2).
Historical perspective
Baron Guillaume Dupuytren did not provide the first description of Dupuytren’s disease; however, his description was the most thorough. Felix Plater provided the first description of palmar contracture in his 1614 work Observationum in hominis affectibus. The original Latin text has been interpreted variably in translation; however, Plater’s anatomic dissections support a role for the palmar fascia in progressive contracture of the palm.18
Henry Cline clearly described the pathologic anatomy and clinical findings present in Dupuytren’s disease in notes that have been discovered in the library of St. Thomas’ Hospital in London.19 Cline’s pupil, Astley Cooper, discussed the condition in his A Treatise on Dislocations and Fractures of the Joints, advocating fasciotomy.20
Guillaume Dupuytren was unaware of these original descriptions when he first described the condition in a lecture delivered on December 5, 1831 at the Hôtel-Dieu, Paris. He described the case of a wine merchant with contracture of the ring and small fingers that he successfully treated with open fasciotomies. His lecture notes were subsequently published in Leçons orales de Clinique Chirurgicale faites à l’Hôtel-Dieu de Paris in 1832.21,22
Basic science and disease process
Basic science
Histopathologic studies have provided the foundation of scientists’ nascent understanding of Dupuytren’s disease. Initial studies have demonstrated not only involvement of the palmar aponeurosis but also absence of adipose tissue, a paucity of sweat glands, and increased vascularity within the affected palm.23 Histologically, nodular rests of myofibroblasts surrounded by dense collagen characterize affected tissue. Molecular analysis reveals a preponderance of type III collagen, which is not typically observed in mature palmar fascia, and increased concentrations of prostaglandins as well as several subtypes of transforming growth factor-β (TGF-β).24,25 DNA microarray analysis comparing diseased and control palmar fascia has revealed a host of gene expression changes, including both up- and downregulation of expression. These include genes previously implicated in the pathogenesis of Dupuytren’s disease, including fibronectin, tenascin C, TGF-β, collagen III, IV, and VI, as well as novel genes, including musculoaponeurotic fibrosarcoma oncogene homolog B (MafB).26 Although the intricate interrelationship between these gene expression patterns remains a field for future inquiry, this knowledge has provided potential clinical targets for pharmacologic intervention in halting or potentially preventing Dupuytren’s disease.
In 1959, Luck described the pathogenesis of Dupuytren’s contracture in pathologic terms consisting of proliferative, involutional, and residual phases. This description has provided a framework within which molecular advances may be analyzed as well as a foundation for clinicians’ understanding of disease progression. The proliferative phase is characterized by nodule formation within the palmar fascia and biochemically by increased fibrinolytic activity. At this stage, fibroblasts differentiate into myofibroblasts and comprise the majority of nodular architecture. Myofibroblasts are fibroblastic in origin; however, they contain an actin microfilamentous structure analogous to smooth-muscle cells. These actin microfilaments are arranged in bundles oriented along the long axis of the cell and communicate with the extracellular matrix fibronectin, thereby allowing transmission of intracellular contractile forces to the extracellular tissues. Marked nodular thickening and signs of early joint contracture characterize the involutional phase. Throughout the involutional phase, type III collagen is synthesized and the myofibroblasts reorient along the lines of tension within the palm. Type III collagen deposition continues and is gradually replaced with type I collagen throughout the residual phase. Myofibroblasts have largely disappeared by the residual phase, resulting in a relatively hypocellular amalgam of type I and type III collagen.27–29
The inciting event resulting in myofibroblast proliferation remains unknown. Authors have suggested multiple hypotheses, including trauma, focal ischemia, and a host of growth factor and cytokine aberrations. Murrell et al. hypothesize that ischemia within the palmar fascia results in the generation of free radicals that damage the surrounding tissue. This damage incites repair with the deposition of collagen and the proliferation of myofibroblasts, and leads to further ischemia and disease progression.30 Mechanical stress has been shown to upregulate myofibroblast differentiation and supports the traumatic theory.31 Myofibroblast proliferation is also stimulated by a number of growth factors and cytokines, including TGF-β2 and inhibited by platelet-derived growth factor (PDGF), basic fibroblastic growth factor, interleukin-1α, and interleukin-1β.32 Actin expression may also play a pivotal role in the progression of Dupuytren’s disease and is increased by TGF-β2 and degreased by PDGF-BB.33 The expression of androgen receptors within Dupuytren’s nodules has also raised the question of hormonal influence on disease development and progression. Myofibroblasts from Dupuytren’s patients stimulated with 5α-dihydrotestosterone demonstrated higher rates of proliferation than myofibroblasts from control subjects, suggesting that androgens may play a role in disease progression. This theory gains support from the male predominance of the disease. Ultimately, none of these theories and biomolecular interrelationships is mutually exclusive and Dupuytren’s disease may very well represent the culmination of a host of environmental and cellular events in a genetically prone individual.
Disease process
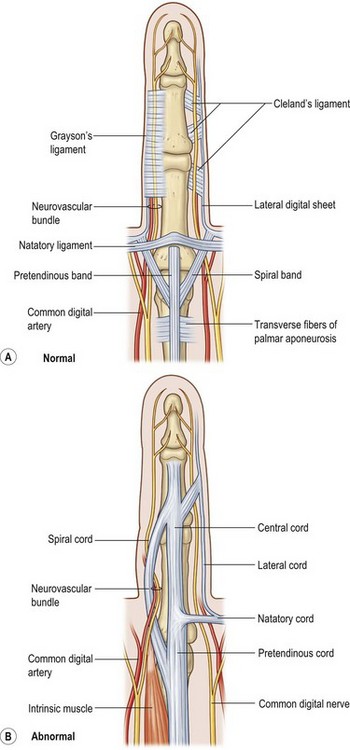
The net effect of these cellular and pathologic mechanisms is the transformation of normal palmar and digital fascial structures into fibrotic diseased cords. Each normal anatomic structure within the palmar and digital fascial system maintains a pathologic correlate. Diseased components may be further subdivided into palmar, palmodigital, digital, first webspace, and hypothenar cords (Table 17.1).
Table 17.1 Fascial anatomy of Dupuytren’s disease
| Diseased structure | Anatomic origin | Clinical significance |
|---|---|---|
| Palmar cords | ||
| Pretendinous cord | Pretendinous band | MCP joint flexion contracture |
| Vertical cord | Vertical fibers of McGrouther or septa of Legueu and Juvara | Causes painful triggering |
| Palmodigital cords | ||
| Spiral cord | Pretendinous band, spiral band, lateral digital sheet, Grayson’s ligament | Displaces the neurovascular bundle medially and superficially (spiral nerve) |
| Natatory cord | Natatory ligament (distal fibers) | Webspace adduction contracture |
| Digital cords | ||
| Central cord | Pretendinous cord (digital extension) | PIP joint flexion contracture |
| Retrovascular cord | Retrovascular band of thomine | PIP and DIP joint flexion contracture; prevents full correction of PIP joint contracture |
| Lateral cord | Lateral digital sheet (often closely associated with pretendinous and natatory cord) | PIP and DIP joint flexion contracture; displaces neurovascular bundle medially |
| Abductor digiti minimi cord | Abductor digiti minimi tendon | PIP joint flexion contracture |
| Thumb and first webspace cords | ||
| Proximal commissural cord | Proximal commissural ligament | First-web adduction contracture |
| Distal commissural cord | Distal commissural ligament | First-web adduction contracture |
| Thumb pretendinous cord | Pretendinous band | MCP joint flexion contracture |
MCP, metacarpophalangeal; PIP, proximal interphalangeal; DIP, distal interphalangeal.
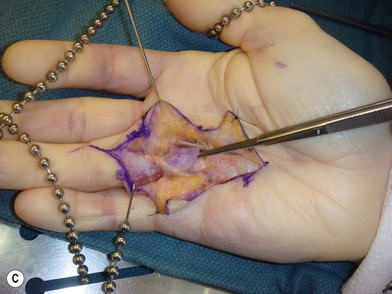
Within the palm, involved pretendinous bands become pretendinous cords. These cords course distally and insert into the deep surface of the dermis at the distal palmar crease, resulting in pitting of the palmar skin. They are also continuous with the retrovascular fascial structures and contribute to formation of the spiral cord.13 The remaining fibers contribute to the formation of the central cord within the finger itself. The pretendinous cord is the primary contributor to MCP flexion contracture and is located superficially, leaving the deeper neurovascular structures undisturbed. Dissection within the palm, proximal to the distal flexor crease, is therefore relatively safe with respect to neurovascular bundles. The vertical fibers of McGrouther and the septa of Legueu and Juvara also become involved and may result in painful triggering with extension of the finger following full flexion (Fig. 17.4).
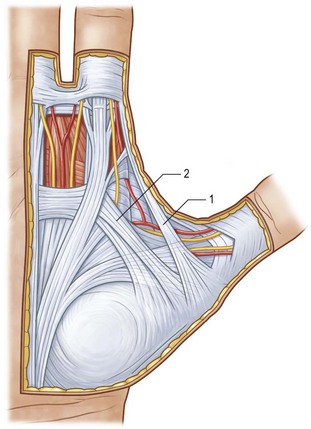
Two key cords are located within the palmodigital region. The spiral cord forms from the diseased confluence of the pretendinous band, spiral band, lateral digital sheet, and Grayson’s ligament. The spiral cord wraps around the neurovascular bundle, displacing the digital nerve and artery medially, superficially, and proximally, placing them at risk for injury during skin incision and dissection (Fig. 17.5). The spiral cord not only displaces the neurovascular bundle, but also results in significant PIP joint contracture.34 The natatory ligament, which traverses the palm transversely at the level of the metacarpal heads, is transformed into the natatory cord. The natatory cord results in webspace adduction contracture.
Within the finger, involved pretendinous bands become central cords. These cords are contiguous proximally with the pretendinous cords of the palm and insert laterally on the radial and ulnar side of the PIP joint and on the flexor tendon sheath of the middle phalanx. The central cord acts in concert with the spiral cord, contributing to PIP joint contracture. Additionally, the retrovascular band of Thomine becomes the retrovascular cord, which contributes to PIP and distal interphalangeal (DIP) joint contracture. The lateral digital sheet may also become involved in disease progression as the lateral cord, contributing to the spiral cord and resulting in PIP and DIP joint contracture.35
The first webspace may also be involved in Dupuytren’s disease. The proximal and distal commissural ligaments, which represent the radial extension of the proximal transverse fibers of the palmar aponeurosis and the natatory ligament, respectively, become the proximal and distal commissural cords. These cords contribute to first-webspace adduction contracture. A thumb pretendinous band, analogous to the longitudinal pretendinous fascial structures of the fingers, evolves into a pretendinous cord and results in MCP joint contracture (Fig. 17.6).
Diagnosis/patient presentation
Clinical presentation
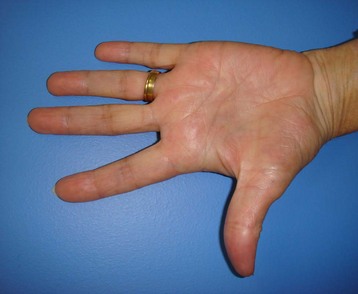
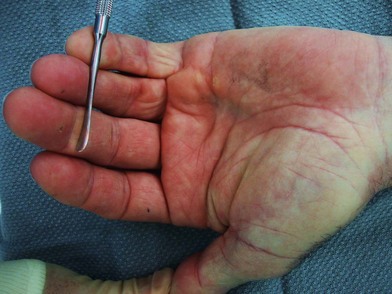
Patients with Dupuytren’s disease present throughout the spectrum of disease progression, and diagnosis is made based upon history and clinical examination. The characteristic progression from nodule to fibrotic cord with contracture of the affected palm and digits is pathognomonic for Dupuytren’s disease. Onset is typically insidious with progressive contracture and disability developing over the course of years. The earliest signs of disease consist of pitting or dimpling of the palm with distortion of the distal palmar crease resulting from connections between the dermis and longitudinal fibers of the palmar aponeurosis13 (Fig. 17.7). Distortion of the skin creases can appear as a deepening of the crease or widening of the crease (Hugh Johnson sign).36 These skin changes are typically the first signs of the disease, preceding the development of nodules. Nodules develop within the palm and are the palpable manifestation of myofibroblast proliferation. These nodules are most commonly located within the ulnar aspect of the palm overlying the ring-finger ray at the level of the distal palmar crease and at the PIP joint level within the finger. Nodules may develop anywhere within the finger, palm, and, rarely, as proximal as the wrist. The nodules are typically painless; however, some patients note discomfort, particularly with grip, and a sensation of tension within the palm.
Disease progression is characterized by the development of longitudinal cords, most commonly in the ulnar aspect of the palm and extending distally into the fingers. The overlying skin is typically undisturbed in the proximal palm and increasingly adherent to the underlying cord distally (Fig. 17.8). The ring finger is most commonly affected, followed in prevalence by the small finger, thumb, middle, and index fingers. A soft palpable fullness immediately adjacent to the cord at the level of the MCP joint may indicate displacement of the neurovascular bundle by a pathologic spiral cord (Short–Watson sign).34
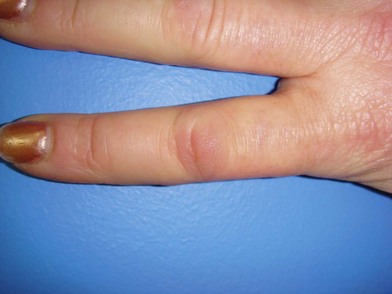
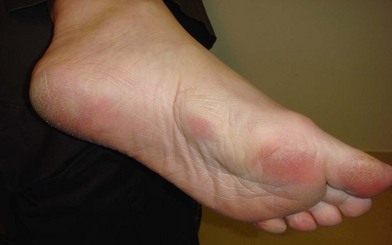
Patients may also present with Garrod’s nodules, or knuckle pads located dorsally over the PIP joints (Fig. 17.9). Garrod’s nodules suggest a higher likelihood of bilateral hand involvement; however, their presence does not suggest stage or severity of disease, nor do they result in any functional limitation themselves.37 Knuckle pads have been reported in 15% of patients with Dupuytren’s disease and may occur in isolation.38,39 Knuckle pads suggest a higher likelihood of concomitant fibromatoses, including Peyronie’s (penile fibromatosis) and Ledderhose’s disease (plantar fibromatosis). Peyronie’s disease affects the tunica albuginea of the corpora cavernosa of the penis and is present in 1–3% of patients with Dupuytren’s disease.40,41 Fibrosis develops as a well-circumscribed plaque, usually on the dorsum of the penis. The disease is generally painless; however, discomfort may occur with erection.42 Plantar fibromatosis is present in 5–20% of patients with Dupuytren’s disease, presenting as a nodular thickening within the nonweight-bearing (arch) portion of the sole of the foot43 (Fig. 17.10). These lesions are typically asymptomatic; however, they may produce discomfort or even severe pain with ambulation in a select number of cases.44,45 Contracture of the toes does not develop. Additional presentations of Dupuytren’s disease include triggering secondary to involvement of the vertical palmar septa, and an isolated tender palmar nodule whose discomfort will typically dissipate with time.
Several other lesions have been reported in association with Dupuytren’s disease; however, no clear relationship has been established. Matev reported involvement of the auricular concha.46 Allen reported the presence of nodules within the tensor fascia lata in patients with Dupuytren’s disease, and Hueston has described involvement of the Achilles tendon.44,47