I. SKIN EMBRYOLOGY
A. Epidermis: Ectoderm
B. Dermis: Mesoderm
C. Other cells
1. Melanocytes: Neural crest
2. Merkel cells: Neural cells
3. Langerhans cells: Mesenchymal
II. SKIN HISTOLOGY
A. Epidermis
1. Keratinocytes
a. Primary cell in epidermis
b. Start in basal layer (stratum germinativum or basale) and make their way to surface becoming a dead cornified layer (stratum corneum).
2. Melanocytes
a. Found in basal layer
b. Protect against ultraviolet (UV) radiation
3. Merkel cells: Mechanoreceptors
4. Langerhans cells: Antigen-presenting cells in stratum spinosum
B. Dermis
1. Cell types: Fibroblast, macrophage, and mast cell
2. Papillary dermis
a. Similar thickness to epidermis
b. High content of type III collagen, less type I
c. Site of collagenase activity.
d. Intertwines with the rete ridges of the epidermis.
e. Contains terminal networks of Meissner corpuscles and capillaries.
3. Reticular dermis
a. Majority of the dermal layer
b. Mostly type I collagen bundles with elastic fibers between
c. Contains roots of the hair, sebaceous glands, sweat glands, receptors, nails, and blood vessels.
4. Tissue components
a. Collagen
i. Tensile strength
ii. Type I to type III—4:1 ratio in adult skin
iii. Immature scar type I to type III—2:1 ratio in adult skin.
b. Elastin
i. Interdigitates with collagen
ii. Important in skin recoil and decreases with aging
iii. Composed of the protein fibrillin
c. Ground substance
i. Noncellular component of extracellular matrix with fibers
ii. Composed of glycosaminoglycans (hyaluronic acid and proteoglycans)
______________
*Denotes common in-service examination topics
A. Generally grouped into three types (listed from most common to least)
B. Basal cell carcinoma (BCC)
C. Squamous cell carcinoma (SCC)
D. Melanoma
1. The ratio of BCC to SCC to melanoma is ≈40:10:1
2. Incidence of all three types is increasing; fortunately, the more common types (BCC and SCC) are far less aggressive than melanoma
3. More than 20% of the US population develops a skin cancer during their lifetime
4. Each year in the United States, there are more new cases of skin cancer than combined new cases of breast, prostate, lung, and colon cancers
BASAL CELL CARCINOMA (BCC)
I. EPIDEMIOLOGY
A. Incidence
1. BCC is the most common skin cancer, accounting for ≈80% of all skin cancers.
2. Roughly 2.8 million new cases per year in the United States.
B. Risk factors
1. Sun exposure (increased with lower latitudes, high altitude): 36% of BCCs originate from the area of previously diagnosed actinic keratosis (AKs), but have distinct cells of origin.
2. Advancing age
3. Fair complexion
4. Long-term exposure to psoralens and UVA therapy (i.e., PUVA therapy for psoriasis)
5. Immunosuppression, most commonly seen in transplant patients
6. Nevus sebaceus of Jadassohn, a superficial skin lesion typically in the head and neck regions, presents as an irregular, raised, yellow to pink, non–hair-bearing raised mass. They are usually present at birth or develop in early childhood, and approximately 15% undergo malignant transformation to BCC.
7. Arsenic exposure
8. Syndromes associated with BCC
a. Basal cell nevus syndrome (Gorlin’s syndrome)
i. Autosomal dominant inheritance
ii. Multiple nevi/lesions often seen early in childhood with malignant degeneration more likely by the age of puberty.
iii. Skin pits on palms and soles, jaw cysts (odontogenic keratocysts), rib abnormalities, mental retardation
b. Xeroderma pigmentosum (XP): Patients have increased incidence of BCC, SCC, and malignant melanoma (see above in melanoma section)
c. Albinism
II. BCC DISEASE BIOLOGY AND CHARACTERISTICS
A. Basal keratinocytes are the cell of origin, residing in the basal layer of the epidermis at the dermoepidermal junction.
B. No universal clinical precursor lesion
C. BCC is most common in areas with high concentrations of pilosebaceous follicles and thus >90% are found on the head and neck.
D. Metastasis is rare—termed “barely a cancer” by some researchers
E. Morbidity is caused by invasion of the tumor into underlying structures, including the sinuses, orbit, and brain. Typically, only a problem if neglected for many years.
F. Types of BCC
1. Nodular BCC
a. The most common type, usually presenting as a single lesion consisting of pearly papules with telangiectasias, pruritus, and occasional bleeding.
b. Lesion breakdown over time leads to nodulo-ulcerative BCC (“Rodent ulcer”).
c. Histology demonstrates palisading nuclei.
a. Slow-growing, erythematous, with minimal induration, and located primarily on the trunk.
b. It is easily confused with other scaly, eczematous dermatoses.
c. The lesions are shallow with a characteristic horizontal growth pattern and often present in multiples.
3. Morpheaform (sclerosing, fibrosing) BCC
a. Flat, often yellowish or hypopigmented, sometimes resembling scars or normal skin.
b. The true extent of the lesion is usually greater than the clinical appearance.
c. There is a high incidence of recurrence or incomplete excision due to “finger-like” extensions.
d. Margins of 1 cm or Mohs extirpation is warranted.
4. Pigmented BCC: Similar to nodular BCC; easily confused with melanoma due to its deep pigmentation and nodularity
5. Adnexal BCC
a. Uncommon and found in older individuals.
b. Tumors arise from sweat glands, and although they exhibit slow growth, they are locally invasive, with a high incidence of local recurrence.
III. TREATMENT OF BCC
A. Standard surgical techniques: ≈95% cure rate
1. Wide local excision of BCC: 3- to 5-mm margins for nonaggressive types and 7-mm margins for morpheaform type.
a. Frozen sections may be used to confirm negative margins intraoperatively. False negatives are common. Surgeon must have confidence in pathologist/ laboratory to use this modality.
2. Mohs surgery: Sequential horizontal excision with immediate frozen section testing by dedicated Mohs dermatopathologist
a. *Indications include morpheaform BCC and/or lesions in aesthetically sensitive areas (nose, eyelid, lip, etc.)
b. Advantages are tissue preservation and confirmation of complete excision.
B. Field therapies
1. Curettage and electrodessication can be used for BCC <1 cm that is NOT a recurrent disease or morpheaform type, but leads to a widened scar.
2. Cryotherapy is effective for small BCC over bone or cartilage, tip of nose, or around the eye.
3. Radiation is effective but requires multiple visits. High cure rates (≈90%), but recurrence is relatively common many years (10 to 15) later.
C. Topical Pharmaceuticals
1. Imiquimod: Immune stimulant. FDA-approved only for superficial BCCs, with cure rates between 80% and 90%. The 5% cream is applied 5 times per week for 6 weeks or longer.
2. 5-Fluorouracil (5-FU): Chemotherapy. FDA-approved for superficial BCCs, with similar cure rates to imiquimod. Five percent liquid or ointment is rubbed onto the tumor 2 times per day for 3 to 6 weeks.
D. Adjuvant radiation therapy (after surgery): Useful for advanced, deeply invasive BCC
SQUAMOUS CELL CARCINOMA
I. EPIDEMIOLOGY
A. Incidence
1. Second most common skin cancer after BCC.
2. Roughly 700,000 new cases annually in the United States.
B. Risk factors
1. UV radiation: Sun exposure and tanning booth use; PUVA therapy for psoriasis
2. Chemical exposure, including some pesticides, organic hydrocarbons such as coal tar, fuel oil, paraffin oil, and arsenic (in welding materials)
3. Viral infection: Some types of human papillomavirus (HPV); herpes simplex virus
4. Radiation: Long latency between exposure and disease.
5. Marjolin’s ulcer: SCC arising in a chronic wound (i.e., chronic burn scars and pressure sores) secondary to genetic changes caused by chronic inflammation.
6. Impaired immunity: That is, immunosuppression for transplants and AIDS. Ratio of SCC to BCC in these patients is 2:1.
7. Fitzpatrick skin type
II. SCC DISEASE BIOLOGY AND CHARACTERISTICS
A. Precursor lesions
1. Actinic keratoses (AKs, or solar keratoses)
a. Erythematous macules and papules with coarse, adherent scale
b. Histologically resembles SCC in situ (pre-malignant)
c. AK is considered a precursor lesion; up to 5% progress to SCC; in turn, 65% of all SCC arise from sites of AKs
2. Bowen’s disease (SCC in situ)
a. Exhibits full-thickness cytologic atypia of the keratinocytes
b. Erythroplasia of Queyrat is SCC in situ of the glans penis.
3. Leukoplakia
a. Presents as a white patch on oral or other mucosa.
b. Malignant transformation occurs in 15%.
4. Keratoacanthoma
a. Benign skin tumor that is composed of squamous cells and keratin; may clinically resemble SCC.
b. Etiology is unknown but thought to originate from hair follicles.
c. Typically has a rapid 6-week growth phase followed by involution over the next 6 months. However, can progress to SCC in 5% to 10% of cases.
d. Excision is the treatment of choice; may be difficult to differentiate from SCC histologically.
B. Types of SCC
1. Verrucous SCC: Slow-growing, exophytic, and less likely to metastasize.
2. Ulcerative SCC: Grows rapidly and is locally invasive.
a. Ulcerative SCC has very aggressive growth characteristics, raised borders, and central ulceration.
b. <50% 5-year survival if spread to lymph nodes in the head and neck.
3. Majorlin’s ulcer
a. Arise from chronic wounds (burn, pressure ulcer, fistula, osteomyelitis tracks)
b. Commonly metastasize to lymph nodes.
III. SCC TREATMENT OPTIONS
A. Standard surgical techniques: 90% to 95% cure rates; similar to BCC options
1. Wide local excision of SCC: 5- to 10-mm margins are usually sufficient. Frozen sections may be used to confirm negative margins intraoperatively.
a. If <2 cm, low grade and extends to dermis, 4-mm margin
b. If >2 cm, grade 2 to 4, high risk or extension into fat, 6-mm margin
2. Mohs surgery: Sequential horizontal excision with frozen section testing. Highest cure rate for SCC: 94% to 99%.
a. Indications, include recurrent, high-risk SCC, and/or lesions in aesthetically sensitive areas (nose, eyelid, lip, etc.)
b. Advantages are tissue preservation and confirmation of complete excision.
B. Field therapies
1. Curettage, electrodessication, and cryotherapy are used much less in SCC treatment than in BCC treatment, because of higher risk associated with missed deep tumor portions, and the risk of scarring obscuring SCC recurrences.
2. Radiation is reserved for unresectable lesions or for the very elderly. Cure rates vary widely. Cosmetic damage and long-term risk of radiation must be considered.
3. Pharmaceuticals are being investigated for topical application, but not currently recommended for invasive SCC.
1. Indicated for clinically positive (palpable) nodes.
2. FNA: Confirm spread of SCC to palpable lymph node.
3. ELND: Indicated for a tumor extending down to parotid capsule or a large lesion contiguous with a draining nodal basin.
4. SLN biopsy: Considered for high-risk SCC without palpable nodes (controversial).
D. Adjuvant radiation therapy: Used postexcision for high-risk cutaneous SCC.
MELANOMA
I. EPIDEMIOLOGY
A. Incidence is increasing, faster than any other cancer in Western world
1. 2% to 3% increase in incidence per year in the United States as of 2009.
2. 75,000 new cases predicted to be diagnosed in the United States in 2012.
3. Lifetime risk in general population is 2% for children born today.
4. Less than 3% of all skin cancers, but cause of 75% of skin cancer-related deaths.
5. Prognosis of metastatic disease has changed little in past 40 years (unlike many other cancers).
B. Risk factors
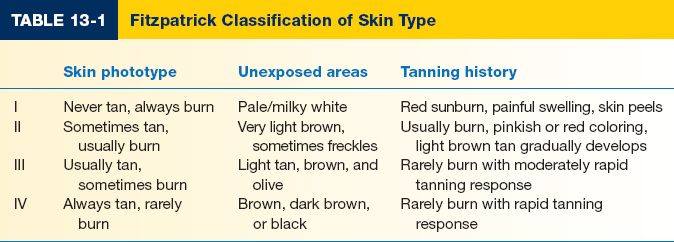
1. Phenotypic include fair skin (Fitzpatrick I and II) (Table 13-1), freckling, light eye color, and light hair color (stronger risk factor than eye color). Darker skin is protective against melanoma.
2. Geographic: High altitudes, lower latitudes have increased UV exposure, and therefore increased risk.
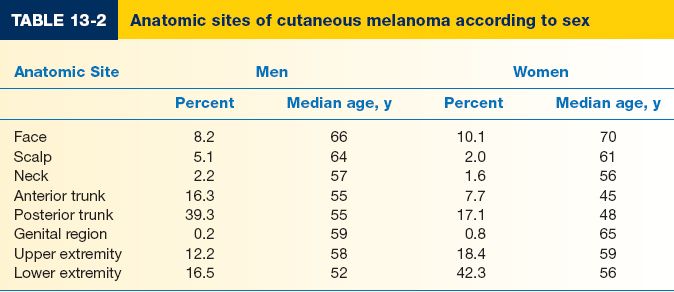
3. Gender: Females have lower risk and better prognosis; however, gender-based differences in risk are lessenings (Table 13-2). Lower extremity is the most common site in females; males more commonly have lesions on the head and trunk.
4. Race: Incidence is lower, but prognosis is worse for African-Americans, due to delayed diagnosis and/or worse disease subtype.
5. Affluence: Unlike most cancer types, higher socioeconomic status correlates with higher risk.
6. History of UV radiation exposure (both UVA and UVB): Evidence for direct causality is less clear than for other skin cancer types. A history of blistering sunburns, particularly in early life, correlates to increased risk of some melanoma types.
7. Previous melanoma is a strong predictive factor and confers a 3% to 5% chance of developing a second melanoma.
8. Family history: Vast majority of melanomas are sporadic; however, some hereditary forms exist (see also Genetics section below).
a. Familial melanoma (aka hereditary melanoma): Two or more cases of melanoma in first-degree relatives may indicate familial melanoma, autosomal dominant transference with variable penetrance.
b. Dysplastic nevus syndrome (also known as familial atypical multiple mole and melanoma [FAMMM] syndrome): Patients have a first- or second-degree relative with malignant melanoma and typically have at least 50 melanocytic nevi. Mutations in CDKN2A typical. Patients need vigilant screening.
c. Xeroderma pigmentosum (XP)
i. Heterogeneous group of syndromes; due mutations in various DNA repair genes.
ii. DNA damage by UV leads to early death secondary to metastatic spread of skin tumors.
iii. Typically presents in childhood with multiple BCCs; SCCs and melanomas typically cause death.
iv. Restriction from sunlight exposure is mandatory, with aggressive surveillance/treatment of skin lesions.
II. MELANOMA DISEASE BIOLOGY AND CHARACTERISTICS
A. Precursor lesions
1. Melanoma is caused by multiple processes leading to malignant transformation of melanocytes.
2. Congenital nevi
a. Malignant potential is more dependent on histology than on size.
b. Giant hairy nevi: Confer a 5% to 20% lifetime risk of melanoma (difficult to predict risk accurately due to variability in size/location); prophylactic excision (often serially) is recommended
3. Acquired melanocytic nevi
a. Typically appear at 6 to 12 months of age; usually <5 mm
b. Increase in number through the fourth decade then slowly regress.
c. The greater the number of nevi, the greater the chance of melanoma.
4. Dysplastic or atypical nevi
a. Often appear in puberty
b. Larger than common nevi (5 to 12 mm)
c. Commonly found in covered areas
d. May represent a precursor lesion and/or marker for increased risk for melanoma development.
5. Melanoma in situ / atypical junctional melanocytic hyperplasia (AJMH) Also termed “lentigo maligna”; Hutchinson freckle
a. Melanoma precursor lesion; no penetration of atypical cells beyond epidermal junction.
b. May arise within dysplastic nevi
c. Needs to be fully excised; 5-mm margins are recommended, but re-excision is often needed.
6. Spitz nevus
a. Benign lesion most commonly found in children and young adults (formerly called juvenile melanoma). NOT a melanoma precursor lesion.
b. Presents as a well-circumscribed, raised lesion with variable pigmentation.
c. Despite the lack of malignant potential, it is very difficult to distinguish histopathologically from melanoma.
d. Recent data indicate that Spitz nevi have mutations in the HRAS gene, distinct from the BRAF/NRAS mutations seen in melanoma.
B. Genetic mechanisms
1. p16/CDKN2A gene: Tumor suppressor gene that is mutated or deleted in the majority of melanoma cell lines; mutations found in some familial melanomas.
2. CDK4 gene: Cell cycle regulator-like CDKN2A; plays a role in melanoma progression in a small proportion of familial and sporadic melanomas.
3. MC1R gene: Pigmentation gene; certain isoforms correlate with fair skin/poor tanning ability as well as increased risk of melanoma.
C. Classification of melanoma types
1. Superficial spreading melanoma
a. Most common type, ≈70% cases
b. Intermediate in malignant potency
c. Most likely to arise from a preexisting nevus
d. Affects both genders equally
e. Median age at diagnosis is 50 years
f. Upper back in men and lower legs in women are most common sites
g. Irregular, asymmetric borders with color variegation
h. Radial growth phase early, vertical growth phase late
2. Nodular melanoma
a. Second most common: 15% to 30% cases
b. Most aggressive type
c. Typically do not arise from preexisting nevi
d. Men are affected twice as frequently as women
e. Median age at diagnosis is 50 years
f. No clear association with sunlight exposure
g. Typically bluish-black, with uniform, smooth borders
h. 5% are amelanotic—associated with a poorer prognosis because of delayed diagnosis
i. Vertical growth phase is a hallmark feature; no radial growth
3. Lentigo maligna melanoma (LMM)
a. 10% to 15% of cutaneous melanomas
b. Least aggressive type
c. Most clearly associated with sunlight/UV exposure
d. Head, neck, and arms of elderly (sun-exposed areas) typically affected
e. Women are affected more frequently than men
f. The median age at diagnosis is 70 years
g. Usually greater than 3 cm in diameter; irregular, asymmetric with color variegation, areas of regression may appear hypopigmented.
h. Precursor lesion is lentigo maligna or Hutchinson freckle (histologically equivalent to melanoma in situ, or AJMH): radial growth phase only. Transition to vertical growth phase marks development of LMM.
i. Malignant degeneration is characterized by nodular development.
4. Acral lentiginous melanoma
a. 2% to 8% of melanomas in Caucasians, 35% to 60% of melanomas in African-Americans, Hispanics, and Asians
b. Presents in palms, soles, and beneath nail plate (subungual). Must be distinguished from melanonychia, a benign, linear, pigmented streak in the nail, common in African and Asian populations. Due to the risk of melanoma, biopsy of suspect lesions should be performed.
c. Median age at diagnosis is ≈60 years
d. Irregular pigmentation, large size (>3 cm) common
e. Most common site is great toe or thumb
f. Long radial growth phase, transition to vertical growth phase occurs with high risk of metastasis.
1. Mucosal melanoma
a. Mucosal melanomas represent <2% of melanomas, most commonly presenting within the genital tract, anorectal region, and head and neck mucosal surfaces.
b. Difficult to detect; typically advanced at the time of diagnosis with poor prognosis.
c. Radical excision is of questionable benefit.
2. Ocular melanoma
a. Represent 2% to 5% of melanomas (most commonly noncutaneous melanoma)
b. Interference with vision leads to earlier diagnosis.
c. Melanomas of iris are similar to cutaneous melanomas in genetics/behavior; melanomas of the posterior uvea act more like mucosal melanomas and have a worse prognosis.
d. The eye has no lymphatic drainage; therefore, no nodal metastasis is seen
e. The liver is the main site of metastatic disease
f. Treatment is by enucleation
E. Melanoma with an unknown primary
1. Represent 3% of melanomas
2. Diagnosis is by exclusion
3. Nodal metastases are the most common presentation
4. Prognosis is similar to metastatic melanomas with a known primary.
III. DIAGNOSIS AND STAGING OF MELANOMA
A. Physical examination is only 60% to 80% sensitive for diagnosing melanoma. Full-body photography to monitor atypical nevi may increase sensitivity.
B. Common clinical features of melanoma lesions: (ABCDE)
1. Asymmetry
2. Border irregularity
3. Color variation
4. Diameter >6 mm
5. Enlarging/evolving lesion
C. Diagnosis of primary melanoma is made by histologic analysis of full-thickness biopsy specimens
1. Excisional biopsy is preferred for lesions <1.5 cm in diameter. If possible, excise lesion with 1- to 2-mm margins.
2. Incisional biopsy is appropriate when suspicion is low, the lesion is large (>1.5 cm) or is located in a potentially disfiguring area (face, hands, and feet), or when it is impractical to perform complete excision. Incisional biopsy does not increase risk of metastasis or affect patient survival.
3. Permanent sectioning is used to determine tumor thickness
4. Avoid shave biopsies, since they forfeit the ability to stage the lesion based on thickness.
5. Do not cauterize or freeze the specimen: Tissue destruction makes it impossible to evaluate thickness and margins.
6. Wide local excision for tissue diagnosis can decrease the efficacy of future lymphatic mapping because of disruption of local lymphatics. Biopsy incisions should result in scars parallel to lymphatic drainage.
7. Orientation of biopsy incisions should also take definitive surgical therapy into consideration.
a. Extremity biopsies should use longitudinal incisions.
b. Transverse incisions are sometimes preferable for preventing contractures over joints.
c. Head and neck incisions should be placed within relaxed skin tension lines, keeping facial aesthetic units in mind.
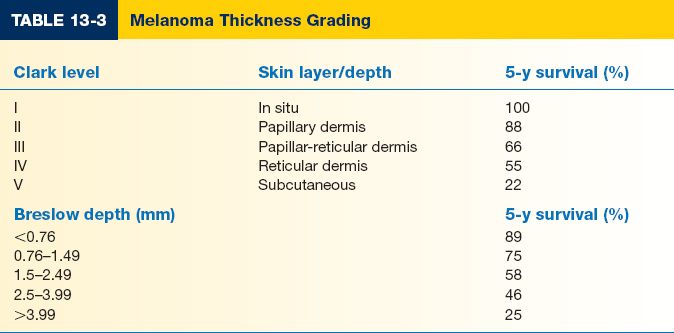
D. Major prognostic factors: Tumor thickness, Nodal status, and Metastases—TNM (Table 13-3)
1. Breslow thickness is reported in millimeters; thus, it is more accurate and reproducible than Clark level and is a better prognostic indicator.
2. Clark level is based on invasion through the histologic layers of the skin; more subjective.
E. Other significant prognostic factors
1. Anatomic location: Trunk lesions generally carry worse prognosis than those on the extremities.
2. Sex: For a given melanoma, women generally have a better prognosis; women are also more likely to have extremity melanomas which carry a better prognosis.
3. Ulceration is a poor prognostic sign
4. Lymph node involvement or in-transit metastases are more significant than any other prognostic factors.
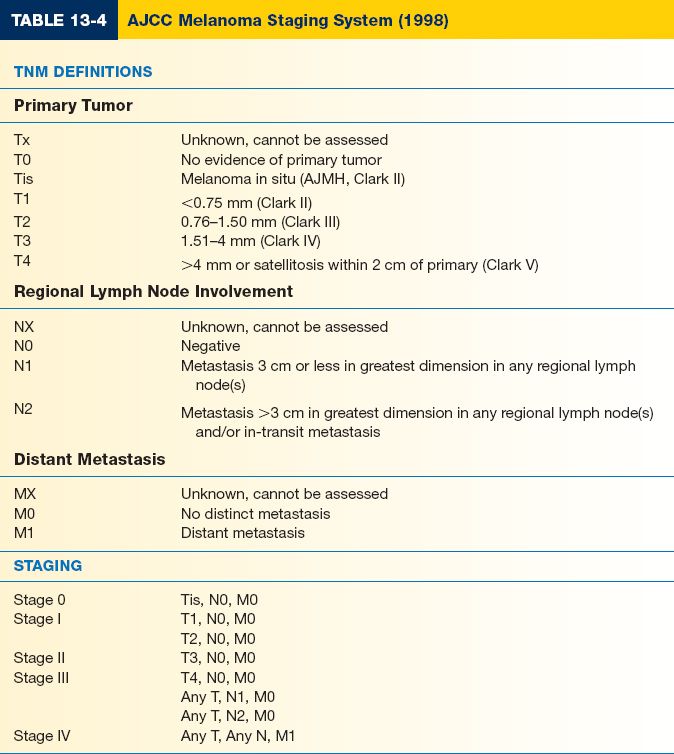
F. The American Joint Committee on Cancer has developed a staging system based on TNM classification (Table 13-4)
IV. MELANOMA TREATMENT
A. Definitive management of melanoma
1. Wide local excision is the treatment of choice.
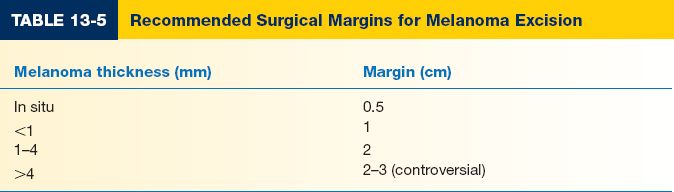
2. Recommended surgical margins depend on tumor thickness (Table 13-5)
3. Subungual melanoma requires amputation proximal to the DIPJ for fingers and proximal to IP joint for the thumb.
B. Management of regional lymph nodes
1. Elective lymph node dissection (ELND) involves removal of clinically negative lymph nodes from the nodal basin. No prospective survival benefit was seen except for a subgroup with 1- to 2-mm (intermediate thickness) melanomas.
2. Sentinel lymph node biopsy (SLNB)
a. In the sentinel node theory, a sentinel node will be the first lymph node seeded by tumor cells, and therefore, excision of sentinel node(s) alone is adequate to determine nodal status. The morbidity of SLNB is considerably less than ELND. Sentinel node(s) can be detected in >90% to 95% of patients. SLNB is now widely considered the standard of care.
b. SLNB is performed in conjunction with wide local excision of the primary tumor. Lymphatic mapping is performed to determine the first lymph node that drains the primary tumor site (sentinel node).
c. SLNB-positive patients undergo staged regional lymphadenectomy and may be candidates for adjuvant therapy.
d. Preoperative nuclear imaging is performed with radiolabeled colloid solution (technetium-99) injected intradermally at the primary tumor. This can be done on the day of or day prior to surgery. Lymphoscintigraphic imaging localizes the sentinel node basin(s) (some tumor sites can drain to multiple basins).
e. In the operating room, a lymphangiography dye (lymphazurin or methylene blue) can be injected intradermally at the periphery of the primary tumor site prior to excision of the primary tumor.
i. Mark edges of the lesion before injection to avoid obscuring them with the dye and take care with the dye because spills are difficult to manage.
ii. Potential sentinel nodes will appear blue when exploring the nodal basin, giving secondary confirmation to localization with Geiger counter detection of Tc99.
iii. Dye injection may briefly interfere with pulse-oximeter readings; alert anesthesiologist at the time of injection.
iv. Caution: Risk of allergy or anaphylaxis with dye injection
f. Following excision of the primary tumor, drapes, instruments, gowns, and gloves are changed and regional lymph node basin(s) identified by lymphoscintigraphy are explored. All radioactive (“hot”) and/or blue nodes are excised.
g. Histologic analysis of sentinel node with immunohistochemical staining identifies micrometastases. Permanent sections are required; frozen sections cannot reliably differentiate normal from neoplastic melanocytes.
C. Surveillance and treatment of melanoma recurrence
1. Asymptomatic patients should be seen every 3 to 4 months for 2 years, then every 6 months for 3 years, and then annually. The most accurate way to detect metastatic disease is to take a thorough history.
2. Chest X-ray and liver function tests (LDH and alkaline phosphatase) are usually sufficient; more extensive work-ups including CT scans have not altered outcomes.
3. Local recurrences typically occur within 5 cm of the original lesion, usually within 3 to 5 years after primary excision; most often this represents incomplete excision of the primary tumor.
4. The most common sites of recurrence are the skin, subcutaneous tissues, distant lymph nodes, then other sites (lung, liver, brain, bone, GI tract).
5. Re-excision is the primary treatment for local, small, isolated lesions
6. Surgery is effective for palliation in patients with isolated recurrences in skin, CNS, lung, or GI tract.
7. Chemotherapy: Complete remission is rare. Decarbazine (DTIC), carmustine, cisplatin, and tamoxifen in combination are most frequently used. Isolated hyperthermic limb perfusion for extensive extremity cutaneous disease (melphalan and tumor necrosis factor) is used at some centers.
8. Cytokine therapy has been demonstrated to produce relatively high levels of tumor response, albeit transient. FDA-approved regimens include interferon-α (IFN-α) for stage III disease and interleukin-2 (IL-2) for stage IV disease; however, these therapies demonstrate little or no improvement in overall survival.
9. Immunotherapies with monoclonal antibodies, tumor vaccines, and modified immune cells have been the subject of active investigation for several decades. Despite a number of dramatic successes, these modalities have yet to prove applicable.
10. Selective cell-signaling inhibitors (e.g., vemurafenib) have recently been developed and can produce dramatic tumor responses in appropriately chosen patients. Increases in survival time, however, do not translate to improve overall survival, as resistant melanomas return with added aggressiveness.
11. Mean survival with disseminated disease is 6 months. Respiratory failure and CNS complications are the most common causes of death.
LESS COMMON SKIN CANCERS
A. Merkel cell carcinoma (MCC)
1. Rare, malignant neuroendocrine tumor arising within the dermis from cells of neural crest origin.
2. Incidence is increasing for unknown reasons; 1,500 cases per year in the United States.
3. Risk factors include age over 65; history of extensive sunlight exposure; fair skin; and immunosuppression (HIV; organ transplants).
4. Recent research has implicated Merkel cell polyomavirus in 80% of MCC cases.
5. Presents as a purple to red papulonodule or indurated plaque; 50% involve the head and neck, 40% the extremities, and 10% the trunk.
6. MCC is aggressive, with radial spread, high local recurrence, and regional and systemic metastasis.
7. Treatment involves local excision with wide (up to 3 cm) margins; SLN biopsy, and postoperative radiation started several weeks later.
8. Poor prognosis; 50% survival at five years.
B. Microcystic adnexal carcinoma
1. Pathophysiology is subject of debate, but many authors support dual follicular and eccrine differentiation.
2. Tumor is invasive and locally destructive.
3. Presents as a white to pink papule–plaque primarily on the head and neck.
C. Sebaceous gland carcinoma
1. Malignant tumor derived from adnexal epithelium of sebaceous glands.
2. Most are periocular; sebaceous gland carcinomas elsewhere are vanishingly rare.
3. Yellowish to pink, slowly growing papulonodule on eyelid (resembles chalazion).
SOFT TISSUE SARCOMAS
I. EPIDEMIOLOGY
A. 6,000 to 7,000 new cases are diagnosed annually in the United States
B. 1% of all malignancies in adults and 15% of those in children
C. 50% are located in the extremities
D. Risk factors
1. The majority of sarcomas have no identifiable predisposing genetic or environmental cause.
2. Radiation exposure
a. Associated with osteosarcomas and malignant fibrous histiocytomas.
b. Typically, there is a 10- to 20-year latency period after exposure.
c. Thorium dioxide (Thorotrast) is a contrast agent used in 1940 to 1950s for radiologic procedures; linked with a high incidence of hepatic angiosarcoma.
3. Chemical exposure: Arsenic, vinyl chloride, and dioxin (contained in the Vietnam War era defoliant Agent Orange)
4. Genetic factors
a. Neurofibromatosis (von Recklinghausen syndrome): 5% lifetime risk of developing neurofibroma or neurofibrosarcoma
b. Mutation in Rb1 tumor suppressor gene: Retinoblastoma (sarcoma of the eye)
c. Mutation in p53 tumor suppressor gene: Li–Fraumeni syndrome (variety of sarcomas)
5. Lymphedema
a. Following surgical procedures, radiation therapy, or parasitic infection; may also arise idiopathically.
b. 10- to 20-year latency for the development of lymphangiosarcoma.
6. Kaposi’s sarcoma: Strongly associated with HIV infection.
II. DIAGNOSIS
A. In contrast to ectoderm-derived carcinomas, sarcomas behave in a similar fashion regardless of the cell of origin.
B. Paucity of local symptoms often leads to advanced disease at diagnosis.
C. A pseudocapsule forms as the tumor expands and compresses adjacent tissue.
D. Major fascial planes typically act as barriers to local invasion.
E. Extremity sarcoma: Generally painless. Delay in diagnosis is common and patients are often erroneously treated for a hematoma or “pulled muscle”
1. Suspicious findings include: Mass >5 cm, enlarging or symptomatic mass, mass present for >4 weeks.
2. MRI is the preferred imaging modality.
3. Pulmonary metastases are the most common location for metastatic disease.
4. Approximately 75% 5-year survival rate
F. Sarcoma of the abdomen or retroperitoneum
1. Can present with vague abdominal complaints: Fullness, early satiety, pain, weight loss, nausea, and vomiting
2. Metastatic disease: Most commonly to liver
3. Palpable mass in 80% of patients at the time of presentation
4. Median survival
a. Primary disease—72 months
b. Recurrent disease—28 months
c. Metastatic disease—10 months
5. Imaging
a. MRI with gadolinium contrast: Best technique for visualizing tumor and relationship to adjacent structures.
b. CT scan: Valuable for evaluating chest/abdomen/pelvis for metastatic disease and as a staging tool.
c. Angiography: For surgical planning
d. Chest X-ray: Evaluates for pulmonary metastasis
6. Biopsy of sarcomas: Performed for extremity lesions <5 cm
III. CLASSIFICATION AND STAGING
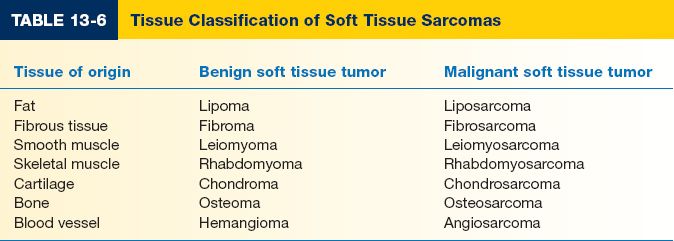
A. Subtypes are named for the cell of origin (Table 13-6). Fibrosarcoma is the most common sarcoma in adults and the second most common in children.
B. Histologic type has little prognostic significance; histologic grade (including frequency of mitotic figures, cellular atypia, and presence or absence of tumor necrosis) is the best for prognosis and therapy.
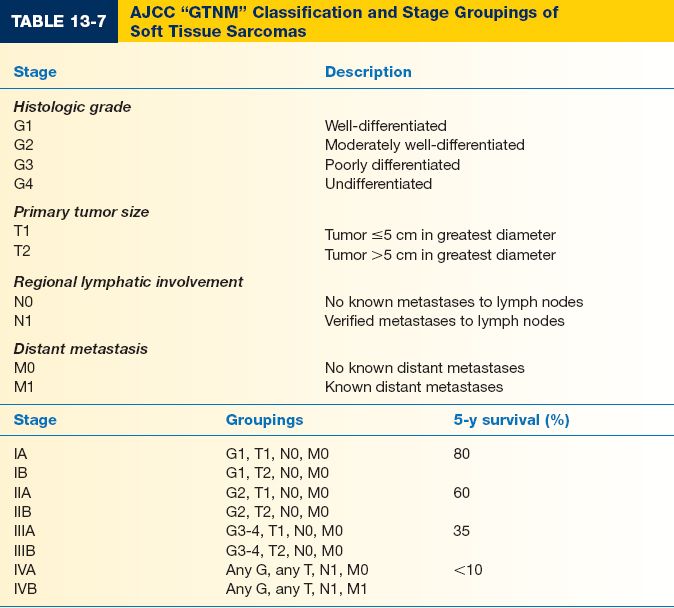
C. Staging criteria (Table 13-7)
1. Histologic grade is the most important prognostic indicator (see above). Low-grade tumors have less than a 15% chance of metastasis; high-grade tumors metastasize in >50%
2. Tumors of larger size are more difficult to grade and have a greater chance of recurrence and dedifferentiation.
3. Nodal and distant metastases are associated with a similar prognosis and are classified as stage IV disease.
4. Five-year survival is on the order of 80% for stage I disease, 60% for stage II, 35% for stage III, and <10% for stage IV
IV. SARCOMA MANAGEMENT
A. Extremities (especially the thighs) are the most common sites for sarcoma
1. Surgery
a. Complete resection with negative margins is the mainstay of treatment.
b. The pseudocapsule should not be entered.
c. Wide local excision (WLE) is the standard of care, with 3- to 5-cm margins of normal tissue proximally and distally. En bloc resection of uninvolved fascial plane with tumor is performed for control of the other margins.
d. WLE is performed after excisional biopsy even if the margins are clear.
e. Major neurovascular structures are generally preserved for low-grade lesions, but are sacrificed and reconstructed as needed for high-grade tumors.
f. There is no survival benefit of amputation compared to limb-sparing procedure.
2. Radiation therapy is not indicated for small (<5 cm) low-grade tumors due to excellent prognosis with WLE alone. It can be used as primary therapy for patients who cannot tolerate or refuse surgery and is also useful as combination therapy for sarcomas up to 10 cm.
3. Chemotherapy is of undetermined benefit in soft tissue sarcoma.
B. Retroperitoneal and intra-abdominal sarcomas have a uniformly poor prognosis. Excision with tumor-free margins is curative, but difficult to achieve. Radiation is rarely used because surrounding organs cannot tolerate therapeutic doses.
PEARLS
1. SCC commonly affects the lower lip and upper eyelid; BCC characteristically affects the upper lip and lower eyelid.
2. Spitz nevus: Looks like melanoma (even under a microscope) but does not metastasize; if it spreads, then it is not a Spitz nevus after all!
3. Perform full-thickness biopsies of pigmented lesions (i.e., punch or excisional) rather than shave biopsy or curettage so that the depth of the lesion can be determined whether it is a melanoma.
4. Merkel cell carcinoma has increasing incidence and behaves much like an aggressive melanoma; unlike melanoma, Merkel cell tumors respond to radiotherapy.
5. Fibrosarcoma is generally not sensitive to chemotherapy or radiation therapy.
QUESTIONS YOU WILL BE ASKED
1. Should you undermine the wound arising from excision of a suspected malignant skin lesion to facilitate wound closure?
No. It will permit spread of malignancy.
2. A 35-year-old breast augmentation patient also mentions a 5-mm, nonhealing wound on the face that has been present for two months. What do you recommend?
Immediate biopsy.
Recommended Readings
Gulleth Y, Goldberg N, Silverman RP, et al. What is the best surgical margin for a basal cell carcinoma: a meta-analysis of the literature. Plast Reconstr Surg. 2010;126(4):1222–1231.
Netscher DT, Leong M, Orengo I, Yang D, Berg C, Krishnan B. Cutaneous malignancies: melanoma and nonmelanoma types. Plast Recon Surg. 2011;127:37E.
Stern RS. Prevalence of a history of skin cancer in 2007: results of an incidence-based model. Arch Dermatol. 2010;146(3):279–282.
< div class='tao-gold-member'>