■ The right adrenal vein is short, often wide, and empties directly into the posterolateral aspect of the inferior vena cava. On occasion, additional right adrenal veins may drain directly into the inferior vena cava or the right hepatic vein. Arterial inflow to the adrenals is less predictable but generally arises as small arteries originating from the renal artery (inferior adrenal artery), the aorta (middle adrenal artery), and the inferior phrenic artery (superior adrenal artery).
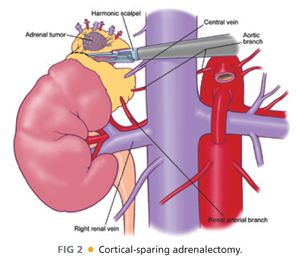
■ Paired small veins may accompany these arteries. It is these anatomic findings that allow for cortical-sparing adrenalectomy in familial pheochromocytoma syndromes, such as von Hippel-Lindau (vHL), neurofibromatosis type 1 (NF1), and multiple endocrine neoplasia type 2 (MEN-2) (FIG 2).
■ Rarely, small rests of adrenocortical tissue may be found at sites near the adrenal bed or within the gonads. This has particular importance when treating corticotropin-dependent hyperadrenocorticism.
PATHOGENESIS
■ Pheochromocytomas, aldosteronomas, and cortisol-secreting tumors produce catecholamines and hormones in an uncontrolled fashion, resulting in potentially life-threatening hormonal sequelae. Some of these tumors are seen in familial cases (MEN, vHL, NF1); most occur sporadically.
NATURAL HISTORY
■ Untreated functional tumors can lead to death and disability. Undiagnosed adrenocortical carcinomas are most often fatal.
PATIENT HISTORY AND PHYSICAL FINDINGS
■ Laparoscopic adrenalectomy is used for small functional and nonfunctional adrenal tumors, the latter being removed because of suspicion of underlying malignancy (either primary or metastatic).
■ Adrenal incidentalomas are incidentally discovered (asymptomatic) adrenal masses, typically picked up on cross-sectional imaging studies performed for some other reason. For example, a patient comes to the emergency room with renal colic and a computed tomography (CT) with stone protocol is performed, revealing a ureteral calculus and an incidentally discovered 4-cm right adrenal mass.
■ Indications for removal an adrenal incidentaloma include (1) a functional lesion because of the risk associated with excess hormonal sequelae; (2) a growing lesion or a lesion greater than 4 cm in diameter because of the risk of adrenocortical malignancy; or (3) an abnormal radiographic phenotype, which can be an indicator of an underlying malignancy.
■ Absolute contraindications to the lateral laparoscopic approach are described in Table 1.

IMAGING AND OTHER DIAGNOSTIC STUDIES
Diagnostic Studies
■ Evaluation of an adrenal incidentaloma should include a 24-hour urine collection for fractionated catecholamines and total metanephrines or plasma metanephrines and normetanephrines.
■ In patients who are hypertensive, regardless of their serum potassium level, plasma aldosterone concentration (PAC) divided by plasma renin activity (PRA) should be calculated to screen for primary aldosteronism. In the case of a high PAC:PRA ratio, confirmation of primary aldosteronism is obtained by salt loading and demonstration of a failure to suppress urinary aldosterone in a 24-hour sample. Autonomous cortisol secretion should be ruled out with an overnight 1 mg or 8 mg dexamethasone suppression test. The demonstration of autonomous cortisol secretion is confirmed by a two-day low-dose dexamethasone suppression test.
■ Other studies available include a 24-hour urinary free cortisol level or demonstration of loss of diurnal variation between a.m. and p.m. plasma cortisol levels.
■ Midnight salivary cortisol levels are also being used with increasing frequency for case detection of cortisol hypersecretion.
■ Studies for estrogen and androgen excess are obtained when clinically indicated but are not routinely done.
Imaging
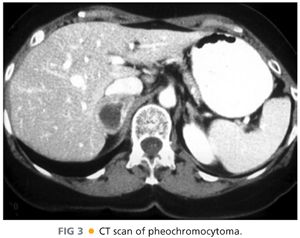
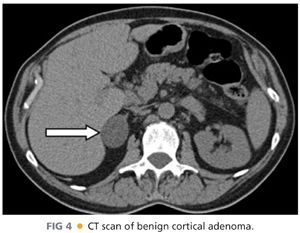
■ Imaging of pheochromocytomas (FIG 3), aldosteronomas, cortisol-secreting tumors, and adrenocortical carcinomas is best performed with CT or magnetic resonance imaging (MRI). Tumors that are round, homogeneous, and low in Hounsfield units on CT scan with rapid washout of intravenous contrast are thought to represent lipid-rich cortical adenomas (FIG 4).
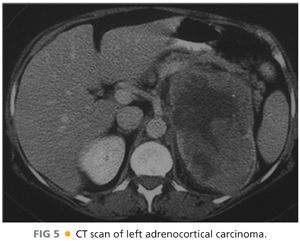
■ Malignancies tend to be large and heterogeneous, with areas of hemorrhage or necrosis associated with high Hounsfield units on CT and delayed washout of intravenous contrast (FIG 5). These lesions appear bright on T2 weighted MRI images.
■ Approximately 25% of lesions 6 cm and greater are likely to be malignant, whereas 6% of lesions between 4 and 6 cm turn out to be primary malignancies. It is for this reason that the 4-cm cutoff is used. This avoids taking out an excessive amount of nonfunctional cortical adenomas while missing very few adrenocortical carcinomas.
■ CT imaging is also valuable in picking up small aldosteronomas, but because of the high incidence of nonfunctional incidentalomas in patients older than 50 years of age, adrenal venous sampling has become the localizing procedure of choice for determining lateralization of an aldosterone-producing adenoma or hyperplasia.
■ Metaiodobenzylguanidine (MIBG) scanning is useful for detecting occult pheochromocytomas, paragangliomas, metastatic disease, and multiple tumors in familial cases.
NONOPERATIVE MANAGEMENT
■ Pheochromocytomas can be managed, albeit less effectively, with medical blockade (α-blockers). Aldosteronomas can be treated with mineralocorticoid receptor blockers, but this is not an ideal choice for younger patients.
SURGICAL MANAGEMENT
Preoperative Considerations
■ Laparoscopic adrenalectomy via a lateral approach is indicated in the following circumstances:
■ All benign, functional adrenal masses less than 6 cm in maximal diameter (aldosteronomas, cortisol-secreting tumors) and pheochromocytomas less than 8 cm in diameter
■ All nonfunctional adrenocortical tumors greater than 4 cm but less than 6 cm in diameter
■ All nonfunctional tumors less than 4 cm in diameter demonstrating interval growth by cross-sectional imaging (CT or MRI)
■ All tumors regardless of size with a worrisome radiographic phenotype (high Hounsfield units on noncontrast CT, poor washout of intravenous contrast on CT, bright image on T2 weighted MRI)
Preoperative Preparation
■
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree