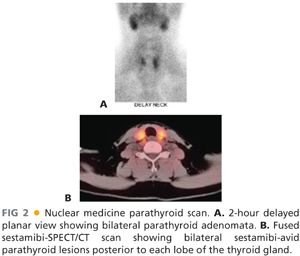
■ Nuclear medicine parathyroid scans using technetium sestamibi as a tracer can accurately identify overactive parathyroid glands about 85% of the time.5 When performed with a concurrent single-photon emission computed tomography (SPECT)/computed tomography (CT) scan, this overlay of functional and structural imaging provides an excellent anatomic map of disease localization invaluable to the operating surgeon (FIG 2A,B). One caveat is that small parathyroid adenomas in close association with the thyroid gland may be difficult to visualize with this imaging technique.
■ Neck CT scan or magnetic resonance imaging (MRI) is occasionally used to image parathyroid glands. Newer CT scan imaging protocols are becoming more widespread, such as 4D CT scans. This takes advantage of the timing of the intravenous (IV) contrast bolus, the vascularity of the parathyroid tumors, and the delayed washout of hyperactive parathyroid lesions.
■ More invasive modalities of parathyroid localization include selective venous sampling for PTH measurement. This technique requires experienced interventional radiologists and is best reserved for the reoperative setting.
■ Imaging prior to reoperative parathyroid surgery is essential to minimize exploration in a scarred operative field and to minimize iatrogenic morbidity. It is ideal to have two concordant imaging studies prior to all reoperative parathyroid surgery (see Chapter 45).
SURGICAL MANAGEMENT
Preoperative Planning
■ Prior to any parathyroid operation, all diagnostic biochemical data should be reviewed to confirm to the surgeon’s satisfaction that a diagnosis of surgically correctable hyperparathyroidism is present in the patient.
■ If an autologous parathyroid transplant is planned, confirmation with the patient as to which upper extremity will be the recipient site should be sought.
■ The wound classification for parathyroid surgery is clean. It is rare for parenteral antibiotics to be indicated prior to parathyroid surgery. Individual patient characteristics (e.g., cardiac valvular lesions, implanted prosthetic hardware), however, should always be considered.
■ Local or general anesthesia may be used.
Positioning
■ The patient is positioned supine on the operating room (OR) table with the arms tucked either at the sides or lying on the abdomen. A sheet, fastened with towel clips, is used to secure the arms next to the patient and to allow for removal of the arm boards from the OR table (FIG 3).
■ If a parathyroid transplant is planned to the patient’s forearm, this arm can be extended from the patient and reprepped and draped at the time of that portion of the procedure (after the parathyroid tissue has been removed from the neck).
■ A towel roll or other small bump is placed behind the patient’s shoulder to aid in extension of the neck.
■ The bed is positioned with the head up, the feet down, and in some slight Trendelenburg. This is known as the semi-Fowler’s position or the beach chair position.
■ Some surgeons will rotate the OR table 90 degrees to have the head of the patient away from the anesthesia providers and thus more accessible to the surgical team.
TECHNIQUES
PLACEMENT OF INCISION
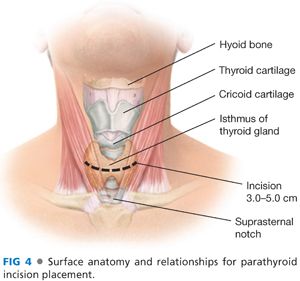
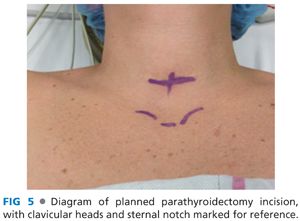
■ A transverse incision is made in the line of a skin crease roughly 1 cm caudal to the cricoid cartilage or two fingerbreadths cephalad to the suprasternal notch. The incision may be between 3 and 5 cm in length and is centered on the midline of the neck (FIG 4). Some surgeons will infiltrate the region of the incision with a local anesthetic combined with epinephrine. Placement of the incision within a natural skin line or crease of the neck is more important for postoperative cosmesis than the length of the incision (FIG 5).
CREATION OF FLAPS
■ Dissection is deepened through the subcutaneous tissues and through the platysma muscle with electrocautery. Subplatysmal flaps are created superiorly, inferiorly, and laterally with a combination of electrocautery and blunt dissection (FIG 6).
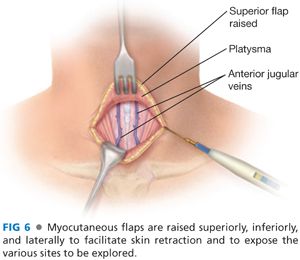
■ Care must be taken to avoid injury to the paired anterior jugular veins. Should a rent be made in one of these veins, it is best to ligate the vein rather than attempt to cauterize the vein.
■ The superior flap should extend to the level of the thyroid cartilage and the inferior flap down to the sternal notch.
ENTRY INTO THE DEEP CENTRAL NECK SPACE
■ The avascular, midline raphe between the sternohyoid and sternothyroid muscles on each side is entered with electrocautery. This midline is best identified by digital palpation of the midline of the underlying trachea. These strap muscles should be separated to expose the underlying thyroid isthmus (FIG 7).
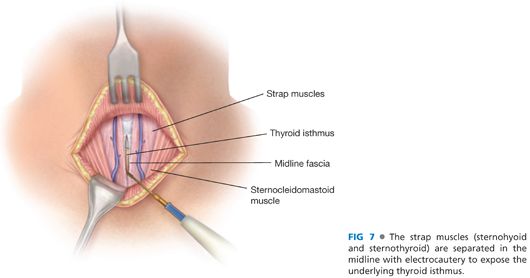
■ The layers of the strap muscles are separated into two by dividing the connective tissue in the layer between the sternohyoid and the sternothyroid muscles. The lateral extent of this dissection is the lateral borders of these muscles and the carotid sheath. This exposes the internal jugular vein, a site from which to draw a baseline blood sample for intraoperative PTH monitoring6 (FIG 8).
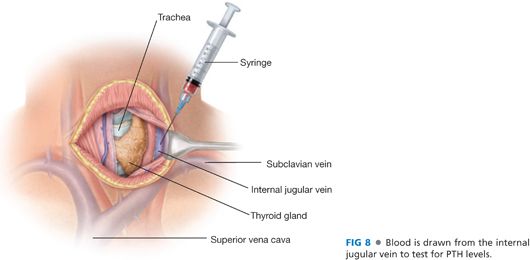
■ An alternate site for PTH sampling is a peripheral venipuncture (often in the lower extremity) performed by the anesthesiologist. If an arterial catheter is present for anesthetic monitoring, it is acceptable to use an arterial blood sample for PTH monitoring.
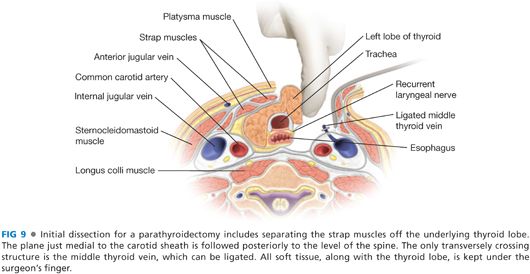
■ The space between the sternothyroid muscle and the thyroid lobe is developed with a combination of blunt dissection and electrocautery. The operation must necessarily begin on one side of the neck, but an identical procedure will be done on the contralateral side of the neck to identify all four parathyroid glands. This space lateral to the thyroid lobe and medial to the carotid artery is developed back to the level of the prevertebral fascia. The crossing middle thyroid vein may need to be divided. This may be accomplished with metallic clips, suture, or through any of the available powered surgical devices (FIG 9). Caution must be taken to avoid searching for parathyroid tissue too soon, as it is possible for the tumor to be more posterior than one has opened, or remaining with either the thyroid or the carotid sheath.
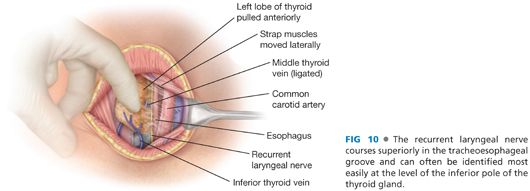
■ The recurrent laryngeal nerve may be identified at this point, coursing superiorly in the tracheoesophageal groove (FIG 10).
IDENTIFICATION OF SUPERIOR PARATHYROID GLANDS
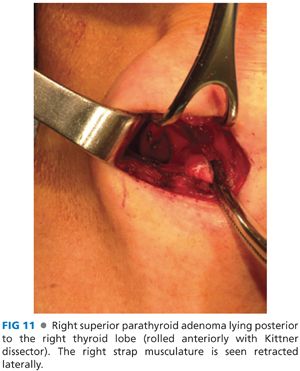
■ The superior parathyroid glands lie posterior to the upper pole of the thyroid lobe. To explore this space, the thyroid lobe is rolled anteromedially using one’s finger or a Kittner dissector. The superior parathyroid glands are posterior to the recurrent laryngeal nerve as it passes underneath the tubercle of Zuckerkandl and just posterolateral to the ligament of Berry (FIG 11). Their position is more constant than that of the inferior parathyroid glands. There is often symmetry between sides of the neck, and if one cannot locate a superior parathyroid gland on one side, it is often advisable to locate it on the contralateral side.
■ Due to limited space for enlargement, superior parathyroid adenomas often grow caudad and ultimately lie in a pseudoectopic position in the tracheoesophageal groove, low in the neck. The blood supply, however, remains in the eutopic position near the upper pole of the thyroid lobe. Caution must be taken elevating these low-lying superior parathyroid adenomas out of the tracheoesophageal groove as they often lie with the recurrent laryngeal nerve draped over them.
■ Ectopic positions for the superior parathyroid glands include low in the tracheoesophageal groove, retroesophageal, retrotracheal, within the carotid sheath, and within the thyroid gland.
■
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








