Unicondylar knee arthroplasty (UKA) is a surgical treatment alternative to total knee arthroplasty (TKA) for replacement of either the medial or lateral tibiofemoral compartment of the knee in selected patients with painful focal arthritis or osteonecrosis.4
 The primary objectives of UKA are pain relief and improvement in lower extremity alignment and function.
The primary objectives of UKA are pain relief and improvement in lower extremity alignment and function.
 UKA implant designs, polyethylene quality, and implant alignment and fixation methods continue to evolve. Adherence to strict surgical indications and appropriate patient selection, combined with meticulous surgical execution, optimizes the functional outcomes and survival rates of UKA.10
UKA implant designs, polyethylene quality, and implant alignment and fixation methods continue to evolve. Adherence to strict surgical indications and appropriate patient selection, combined with meticulous surgical execution, optimizes the functional outcomes and survival rates of UKA.10
 Although UKA has numerous potential clinical advantages, the technical challenges in achieving consistent, accurate component alignment and soft tissue balance have prompted advances in the development and use of computer and robotic technologies.13,15,16,25
Although UKA has numerous potential clinical advantages, the technical challenges in achieving consistent, accurate component alignment and soft tissue balance have prompted advances in the development and use of computer and robotic technologies.13,15,16,25
ANATOMY
 The knee has three compartments: the medial and lateral tibiofemoral compartments and the patellofemoral compartment.
The knee has three compartments: the medial and lateral tibiofemoral compartments and the patellofemoral compartment.
 The alignment of the knee joint is described relative to the mechanical axis of the lower extremity, which is a straight line from the center of the femoral head to the center of the ankle joint.
The alignment of the knee joint is described relative to the mechanical axis of the lower extremity, which is a straight line from the center of the femoral head to the center of the ankle joint.
When the center of the knee lies on the mechanical axis, the knee is in neutral alignment, which allows for appropriate load distribution between the medial and lateral compartments of the knee.
If the mechanical axis passes medial to the knee center, a varus deformity is present; if the axis passes lateral to the knee center, a valgus deformity is present.
 Normal knee joint alignment is defined by asymmetric bony anatomy, which creates a tibiofemoral angle of approximately 5 to 7 degrees valgus, ligamentous tension, and a uniform joint space throughout the medial and lateral compartments through the full range of motion of the knee.19,38
Normal knee joint alignment is defined by asymmetric bony anatomy, which creates a tibiofemoral angle of approximately 5 to 7 degrees valgus, ligamentous tension, and a uniform joint space throughout the medial and lateral compartments through the full range of motion of the knee.19,38
The proximal articular surface of the tibia is oriented approximately 3 degrees varus to the mechanical axis of the tibia.
In the sagittal plane, the tibia is sloped posteriorly approximately 5 to 7 degrees to accommodate femoral rollback during knee flexion.
The distal femoral condyles are oriented approximately 3 degrees valgus to the mechanical axis of the femur and 9 degrees valgus to the anatomic axis of the femur.
In flexion, the medial femoral condyle extends more posteriorly than the lateral femoral condyle.34
 The goal of UKA is to restore the affected tibiofemoral joint line by implanting a prosthesis that matches the thickness of the bone and cartilage lost or resected, thereby correcting or partially correcting the deformity with balanced soft tissues so that there is 1 to 2 mm of laxity through the full range of motion of the knee.
The goal of UKA is to restore the affected tibiofemoral joint line by implanting a prosthesis that matches the thickness of the bone and cartilage lost or resected, thereby correcting or partially correcting the deformity with balanced soft tissues so that there is 1 to 2 mm of laxity through the full range of motion of the knee.
PATHOGENESIS
 The etiologies of articular cartilage degeneration include primary osteoarthritis (OA), osteonecrosis, and arthritis secondary to trauma, infection, or an inflammatory process.
The etiologies of articular cartilage degeneration include primary osteoarthritis (OA), osteonecrosis, and arthritis secondary to trauma, infection, or an inflammatory process.
 The degenerative process causes deterioration and loss of the bearing surface of the knee joint, characterized by cartilage and osteochondral junction breakdown, subchondral microfractures and cyst formation, and increased osseous stresses leading to the development of osteophytes.1
The degenerative process causes deterioration and loss of the bearing surface of the knee joint, characterized by cartilage and osteochondral junction breakdown, subchondral microfractures and cyst formation, and increased osseous stresses leading to the development of osteophytes.1
 When isolated to the medial or lateral tibiofemoral compartment, articular degeneration reduces the joint space in the affected compartment, causing overall malalignment of the knee joint.
When isolated to the medial or lateral tibiofemoral compartment, articular degeneration reduces the joint space in the affected compartment, causing overall malalignment of the knee joint.
 In medial compartment OA with an intact anterior cruciate ligament (ACL), the cartilage loss is typically anteromedial, whereas the posterior tibial and femoral cartilage are preserved.17,39
In medial compartment OA with an intact anterior cruciate ligament (ACL), the cartilage loss is typically anteromedial, whereas the posterior tibial and femoral cartilage are preserved.17,39
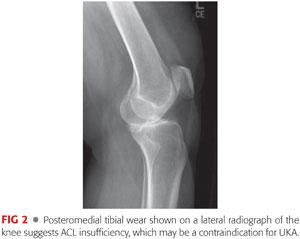
Posteromedial tibial wear suggests ACL insufficiency and may be a contraindication for UKA.
 In lateral compartment OA, the wear is typically on the posterior (flexion) surface of the lateral femoral condyle, with preservation of the distal femoral cartilage.
In lateral compartment OA, the wear is typically on the posterior (flexion) surface of the lateral femoral condyle, with preservation of the distal femoral cartilage.
 Unicompartmental arthritis most commonly involves the medial compartment, causing a varus deformity.
Unicompartmental arthritis most commonly involves the medial compartment, causing a varus deformity.
NATURAL HISTORY
 Cartilaginous degeneration of the knee joint is progressive as described by the Outerbridge grading system.
Cartilaginous degeneration of the knee joint is progressive as described by the Outerbridge grading system.
Grade I: soft superficial discoloration
Grade II: fragmentation less than 1.3 cm2
Grade III: fragmentation greater than 1.3 cm2
Grade IV: erosion to subchondral bone (eburnation)
 Angular malalignment of the lower extremity accentuates stress on the damaged articular cartilage, causing pain, progression of unicompartmental arthritis, and increased angular deformity of the knee.20
Angular malalignment of the lower extremity accentuates stress on the damaged articular cartilage, causing pain, progression of unicompartmental arthritis, and increased angular deformity of the knee.20
For a single-leg stance with a neutral mechanical axis, the load across the medial compartment is approximately 60%. The load increases progressively up to 90% in the presence of a varus deformity of 4 to 6 degrees.19
 In the most advanced stages, the degenerative process leads to ligamentous instability with failure of the ACL perpetuating joint incongruity and the progression of arthritis to the posterior aspect of the tibiofemoral articulation and, at the end-stage of the disease, to the adjacent compartments of the knee.17
In the most advanced stages, the degenerative process leads to ligamentous instability with failure of the ACL perpetuating joint incongruity and the progression of arthritis to the posterior aspect of the tibiofemoral articulation and, at the end-stage of the disease, to the adjacent compartments of the knee.17
However, many cases (approximately 15% to 35%) will never progress beyond one compartment.5
PATIENT HISTORY AND PHYSICAL FINDINGS
 The most common presenting symptom of unicompartmental knee arthritis is activity-related pain confined to the affected compartment and relieved by rest.20
The most common presenting symptom of unicompartmental knee arthritis is activity-related pain confined to the affected compartment and relieved by rest.20
Associated symptoms include swelling, instability, stiffness, and restriction of activity.
Diffuse knee pain and rest pain suggest multiple compartment involvement and more advanced arthritis.
Anterolateral pain with stair climbing, prolonged sitting, or squatting suggests patellofemoral involvement.
Mechanical symptoms such as locking or catching may be related to articular surface irregularity, loose bodies, or meniscal pathology, which are commonly seen in conjunction with arthritis.
Knowledge of response to previous treatments such as nonsteroidal anti-inflammatory drugs (NSAIDs), injections, or bracing is helpful to direct further management.
 Physical examination findings consistent with unicompartmental knee arthritis may include joint line tenderness, joint effusion, crepitus, malalignment, and pseudolaxity.
Physical examination findings consistent with unicompartmental knee arthritis may include joint line tenderness, joint effusion, crepitus, malalignment, and pseudolaxity.
Unicompartmental medial or lateral degeneration may cause varus or valgus deformity about the knee, respectively.
• Medial compartment arthritis amenable to UKA presents with a varus deformity of up to 10 degrees, which is passively correctable toward neutral alignment in zero degrees of knee flexion.
• Lateral compartment arthritis conducive to UKA presents with a valgus deformity of up to 15 degrees, which is passively correctable toward neutral alignment and does not increase in magnitude with further valgus stressing (indicative of incompetence of the medial collateral ligament).
Range of motion and the presence and degree of flexion contracture are assessed.
• UKA requires a minimum of 90 degrees of flexion and no more than a 5- to 10-degree flexion contracture.23
Ligamentous stability must be present for consideration of UKA.
• ACL deficiency without functional instability is not a contraindication for UKA. However, functional ACL instability during activities and posterior tibial wear due to anterior tibial subluxation is a contraindication for UKA.17
Other physical examination considerations include the following:
• Previous incisions
• Patellofemoral or adjacent tibiofemoral tenderness or crepitus
• Body habitus
• Indications of spine or ipsilateral hip pathology
• Neurovascular status
IMAGING AND OTHER DIAGNOSTIC STUDIES
 Preoperative radiographs include weight-bearing anteroposterior (AP), midflexion posteroanterior (PA), lateral, and skyline patellar views to assess tibiofemoral alignment and the condition of the remaining compartments of the knee (FIG 1A–D).
Preoperative radiographs include weight-bearing anteroposterior (AP), midflexion posteroanterior (PA), lateral, and skyline patellar views to assess tibiofemoral alignment and the condition of the remaining compartments of the knee (FIG 1A–D).
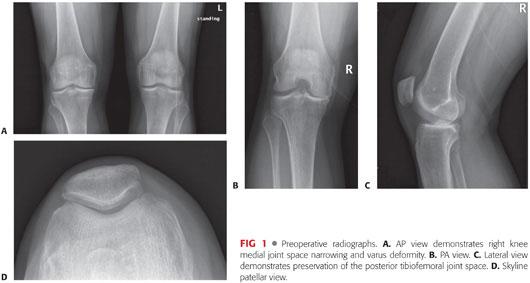
Radiographic findings of unicompartmental knee arthritis may include joint space narrowing, subchondral sclerosis and cystic change, osteophytes, and malalignment of the affected lower extremity.
Small patellar osteophytes or peripheral osteophytes of the other tibiofemoral compartment may be seen in the absence of cartilage wear in those compartments and are not contraindications to UKA.
Large subchondral cysts would exclude UKA due to potentially compromised osseous support.
Tibiofemoral contact is assessed on the lateral radiograph and is an indirect indicator of the functional integrity of the ACL. Concave erosion on the anterior two-thirds of the tibial plateau is indicative of an intact ACL (95% probability). Posterior contact and wear are characteristic of chronic ACL laxity (FIG 2).17
 Some surgeons perform stress view radiographs to identify whether there is occult full-thickness cartilage loss in the unaffected tibiofemoral compartment as well as to determine how correctible the deformity is.
Some surgeons perform stress view radiographs to identify whether there is occult full-thickness cartilage loss in the unaffected tibiofemoral compartment as well as to determine how correctible the deformity is.
 Magnetic resonance imaging (MRI) and computed tomography (CT) are infrequently used in the standard workup of patients suspected of having unicompartmental arthritis, unless there is a significant discrepancy between history and physical examination findings and the findings on plain radiographs.
Magnetic resonance imaging (MRI) and computed tomography (CT) are infrequently used in the standard workup of patients suspected of having unicompartmental arthritis, unless there is a significant discrepancy between history and physical examination findings and the findings on plain radiographs.
DIFFERENTIAL DIAGNOSIS
 Unicompartmental OA
Unicompartmental OA
 Bi- or tricompartmental OA
Bi- or tricompartmental OA
 Osteonecrosis
Osteonecrosis
 Meniscal tear
Meniscal tear
 Osteochondral injury
Osteochondral injury
 Pes anserine bursitis
Pes anserine bursitis
 Iliotibial band syndrome
Iliotibial band syndrome
 Saphenous neuritis
Saphenous neuritis
 Septic arthritis
Septic arthritis
 Hip or spine pathology
Hip or spine pathology
NONOPERATIVE MANAGEMENT
 Multiple nonoperative management options are available for treatment of unicompartmental knee OA, each with varying degrees of success.4 These include the following:
Multiple nonoperative management options are available for treatment of unicompartmental knee OA, each with varying degrees of success.4 These include the following:
Lifestyle modifications including weight loss and low-impact exercise
NSAIDs
Analgesic medications
Physical therapy
Ambulatory assistive devices
Unloader braces
Viscosupplementation injections
Intra-articular corticosteroid injections
Glucosamine and chondroitin sulfate supplements
SURGICAL MANAGEMENT
 UKA is a surgical treatment alternative to TKA or periarticular osteotomy in selected patients with painful unicompartmental knee degeneration.
UKA is a surgical treatment alternative to TKA or periarticular osteotomy in selected patients with painful unicompartmental knee degeneration.
 Adherence to sound indications and patient selection criteria is a key determinant of successful outcome following UKA.
Adherence to sound indications and patient selection criteria is a key determinant of successful outcome following UKA.
Conservative data suggest that 6% to 15% of arthroplasty patients are candidates for UKA, although these numbers may be as high as 30% or more.5,32,37
 Classic indications and prerequisites for UKA include the following:9,10,23,27,35
Classic indications and prerequisites for UKA include the following:9,10,23,27,35
Diagnosis of noninflammatory unicompartmental OA or spontaneous osteonecrosis with symptoms resistant to nonoperative management
Radiographic evidence of preservation of alternate compartments of the knee
No pain and no exposed bone in alternate compartments of the knee
Low-demand patients
Age older than 60 years
Weight less than 82 kg (181 pounds)
A minimum range of motion of 90 degrees
Flexion contracture of less than 5 degrees
Intact ACL
Angular deformity of the knee of a maximum of 10 degrees varus or 15 degrees valgus and partially passively correctable
 Expanded indications for UKA include the following6,30:
Expanded indications for UKA include the following6,30:
Younger, more active patients as a bridge procedure
Moderate obesity (body mass index 35 to 40 or less)
• UKA is used cautiously in the morbidly obese due to more diffuse disease and a potentially increased risk of aseptic loosening and wear (debated in the literature).6,7,11
• Recent studies have not supported a correlation between weight and poor outcome following UKA.11
ACL deficiency, if there is no functional instability and the tibiofemoral contact is anterior17
Asymptomatic grade IV patellofemoral chondromalacia, if the lateral facet and lateral trochlea are spared
 Additional considerations include age and occupational or recreational demands. The decision to proceed with UKA requires a prudent risk–benefit analysis based on multiple factors.24
Additional considerations include age and occupational or recreational demands. The decision to proceed with UKA requires a prudent risk–benefit analysis based on multiple factors.24
 Although the decision to proceed with UKA is most often made prior to surgery, the patient’s suitability for UKA is ultimately confirmed after arthrotomy.
Although the decision to proceed with UKA is most often made prior to surgery, the patient’s suitability for UKA is ultimately confirmed after arthrotomy.
The patient may be consented in advance for possible TKA or combined UKA and patellofemoral arthroplasty in cases where it is not completely clear in advance that UKA is appropriate.
Preoperative Planning
 High-quality radiographs allow for proper preoperative evaluation, including standing AP, midflexion PA, lateral, sunrise, and stress views (at the surgeon’s discretion).
High-quality radiographs allow for proper preoperative evaluation, including standing AP, midflexion PA, lateral, sunrise, and stress views (at the surgeon’s discretion).
 A full-length, weight-bearing AP radiograph from the hip to the ankle is helpful in determining the mechanical and anatomic axes of the lower extremity and assessing unusual bowing or deformity, as well as occult pathology of the hip or ankle, although this radiograph is not used routinely.
A full-length, weight-bearing AP radiograph from the hip to the ankle is helpful in determining the mechanical and anatomic axes of the lower extremity and assessing unusual bowing or deformity, as well as occult pathology of the hip or ankle, although this radiograph is not used routinely.
 Template overlay can be used to estimate component size and bone defects.
Template overlay can be used to estimate component size and bone defects.
Positioning
 The patient is positioned supine on the operating table with a bump, roller, or dynamic leg positioner secured to the table to support the patient’s heel when the knee is flexed.
The patient is positioned supine on the operating table with a bump, roller, or dynamic leg positioner secured to the table to support the patient’s heel when the knee is flexed.
 A thigh tourniquet is applied as proximally as possible on the operative lower extremity.
A thigh tourniquet is applied as proximally as possible on the operative lower extremity.
 Surgical drapes are applied to isolate the sterile surgical field.
Surgical drapes are applied to isolate the sterile surgical field.
 If a tourniquet is used, the lower extremity is exsanguinated by application of an elastic compressive wrap and inflation of the tourniquet.
If a tourniquet is used, the lower extremity is exsanguinated by application of an elastic compressive wrap and inflation of the tourniquet.
Approach
 Note: For purposes of this chapter, the primary emphasis of surgical details will focus on the more common medial UKA.
Note: For purposes of this chapter, the primary emphasis of surgical details will focus on the more common medial UKA.
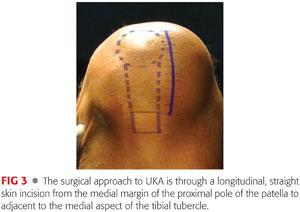
 With the knee in moderate flexion, a skin incision is made from just proximal to the medial margin of the proximal pole of the patella to the medial aspect of the tibial tubercle (FIG 3). The underlying extensor mechanism and joint capsule are exposed.
With the knee in moderate flexion, a skin incision is made from just proximal to the medial margin of the proximal pole of the patella to the medial aspect of the tibial tubercle (FIG 3). The underlying extensor mechanism and joint capsule are exposed.
 The knee joint is accessed via a medial parapatellar arthrotomy from the proximal patella to just medial to the tibial tubercle. Great care is taken along the superior portion of the arthrotomy to avoid damage to the intact cartilage of the trochlea. A cuff of capsular tissue is left intact along the medial border of the patella for later wound closure.
The knee joint is accessed via a medial parapatellar arthrotomy from the proximal patella to just medial to the tibial tubercle. Great care is taken along the superior portion of the arthrotomy to avoid damage to the intact cartilage of the trochlea. A cuff of capsular tissue is left intact along the medial border of the patella for later wound closure.
Alternatively, and less commonly, a lateral incision and lateral parapatellar capsulotomy can be used for UKA of the lateral compartment. The lateral arthrotomy must extend more proximally to allow medial subluxation of the patella.31
In a very tight or muscular knee, extending the incision more proximally, incising the medial capsule transversely for 1 cm just below the vastus medialis muscle, or formalizing a midvastus or subvastus approach may help facilitate surgical exposure.
TECHNIQUES
 Exposure
Exposure
After the arthrotomy, all three compartments of the knee are inspected to confirm the decision to proceed with UKA.
The patella is subluxated laterally as the knee is brought into flexion to achieve exposure of the medial joint.
A portion of the retropatellar fat pad is excised to facilitate visualization.
The coronary ligament is incised at the anterior horn of the medial meniscus and a sharply dissected subperiosteal sleeve of soft tissue that includes the deep medial collateral ligament is elevated from the anteromedial aspect of the tibia along the joint line.
For replacement of the lateral compartment, the coronary ligament is incised lateral to the midline and a periosteal sleeve is elevated from the anterolateral aspect of the tibia, as far as Gerdy’s tubercle.
An osteotome or rongeur is used to remove notch and marginal osteophytes that may cause impingement or interfere with proper collateral ligament balancing.
 Distal Femoral Condyle Resection
Distal Femoral Condyle Resection
With the knee flexed to 90 degrees, an entry hole is drilled in the distal femur 1 cm anterior to the origin of the posterior cruciate ligament (PCL) and just anterior to the intercondylar notch to accommodate an intramedullary femoral alignment and resection guide (TECH FIG 1A).

The intramedullary guide is inserted into the femoral medullary canal, in line with the anatomic axis of the femur. The resection guide is flush with the distal femoral condyle, rotationally parallel to the resected tibial surface, and perpendicular to the tibial shaft (TECH FIG 1B).
A distal femoral cutting block is attached to the intramedullary guide and allows for adjustment of the cut angle relative to the anatomic axis of the femur (TECH FIG 1C).
The anatomic angle of the distal femoral cut is typically 4 to 7 degrees valgus.
An alternative method of distal femoral resection is to use a spacer block technique, which ensures parallelism to the tibial cut.
With retractors positioned to protect the collateral ligament and soft tissues, a narrow, oscillating or reciprocating saw blade is used to cut the distal femoral condyle through the appropriate slot of the cutting block (TECH FIG 1D).
An 8-mm spacer block is inserted into the extension space to ensure adequate spacing without overstuffing or overcorrection.
 Posterior Femoral Condyle Resection
Posterior Femoral Condyle Resection
An insertion handle is attached to a femoral sizing and posterior resection guide to position its flat surface on that of the cut distal femoral condyle (TECH FIG 2A).
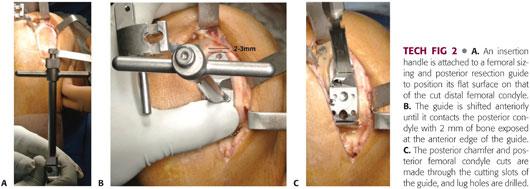
Various guides are trialed to select the proper size, so that when the guide is positioned on the posterior femoral condyle, 2 mm of bone is exposed between the anterior edge of the guide and the cartilage tidemark (TECH FIG 2B). This minimizes the risk of patellar impingement on the femoral component as the knee flexes.
The posterior/chamfer resection guide is secured in position with the posterior surface of the guide parallel to the intended proximal tibial cut. It is lateralized toward the intercondylar notch to centralize the femoral component on the tibia.
The anterior and posterior lug holes are drilled.
The posterior femoral condyle and chamfer cuts are made through the cutting slots in the guide, with a retractor in place to protect the collateral ligament (TECH FIG 2C).
 Proximal Tibial Resection
Proximal Tibial Resection
The anterior aspect of the tibia is exposed in the distal part of the wound from the margin of the tibial plateau to the tibial tubercle.
An extramedullary tibial alignment and resection guide is positioned with its resector stem parallel to the mechanical axis of the tibia in the coronal plane, so that the stem is directly over the medial third of the tibial tubercle proximally and the tibial crest distally.
Although ideally the guide is parallel to the entire tibial crest, this is not possible in the setting of tibial bowing.
With the ankle clamp secured distally, the guide often points to the second metatarsal of the neutrally positioned foot.
The posterior slope of the resection guide is set to mimic the native slope of the tibial plateau.
The sagittal slope should be measured on preoperative radiographs, and if the slope is greater than 7 degrees, the posterior slope should be limited to no more than 7 degrees (TECH FIG 3A).18

With the knee flexed 90 to 100 degrees, a narrow, reciprocating saw blade is used to make a vertical tibial cut in the sagittal plane parallel to and just off the peak of the tibial eminence (TECH FIG 3B).
The cut should not extend deep to the intended level of the horizontal cut as this could cause tibial plateau fracture.
Care is taken to avoid disrupting the ACL or PCL attachment.
With a retractor positioned to protect the collateral ligament and soft tissues, a conservative horizontal cut is made with an oscillating saw blade perpendicular to the mechanical axis of the tibia in the coronal plane and with a slight posterior slope as described (TECH FIG 3C).
The level of proximal tibial resection is guided by the depth of tibial erosion so that the cut removes the sclerotic surface and extends approximately 2 to 4 mm below the deepest part of erosion.
In the presence of large osteophytes and more extreme but partially correctable deformity, less of the tibia (2 mm) is typically resected. When there are minimal osteophytes, slightly more tibial resection (4 mm) may be necessary.
In lateral UKA, tibial resection should be highly conservative.
A tibial alignment rod dropped from the cut tibial plateau surface in alignment with the mechanical axis of the tibia confirms proper orientation of the tibial cut (TECH FIG 3D).
The goal is to produce a parallel relationship between the distal femoral condyle cut and the proximal tibial cut in full extension and flexion.
The meniscus and any remaining osteophytes are removed.
 Balancing the Flexion and Extension Gaps
Balancing the Flexion and Extension Gaps
All retractors and implants are removed.
Gap spacers corresponding to the various tibial articular surface thicknesses are used to assess the flexion and extension gaps.
If the joint space is symmetrically tight in flexion and extension, additional tibial bone is resected or the polyethylene insert is downsized.
If the joint space is symmetrically loose in flexion and extension, the thickness of the polyethylene insert is progressively increased.
If the joint space is asymmetrically tight in extension and acceptable in flexion, additional distal femur is resected or the posterior tibial slope is reduced by removing some anterior tibial bone.
If the joint is asymmetrically tight in flexion and acceptable in extension, the posterior slope of the tibial resection is increased or the femoral component is downsized with resection of additional posterior femoral condyle.
After any adjustment or additional resection, the flexion and extension gaps are rechecked.
Care is taken to not overstuff the operative compartment. One to 2 mm of laxity is appropriate following proper balancing of the flexion and extension gaps. Overstuffing may limit motion and/or overcorrect the alignment and cause overloading of the opposite tibiofemoral compartment.
 Trial Component Insertion and Reduction
Trial Component Insertion and Reduction
To determine proper tibial component size, a tibial sizing tray is positioned on the cut surface of the proximal tibia with the straight edge against the flat sagittal surface along the tibial spine.
The tray size should be selected to maximize coverage of the resected proximal tibial surface while minimizing overhang.
If the tibial component covers the tibial surface from medial to lateral but not completely from anterior to posterior, the component should be positioned on the anterior tibial cortex to try to reduce the risk of anterior tibial subsidence.
The properly sized trial tibial baseplate is positioned onto the cut surface of the tibia, and the impactor engages the central fin into the bone so that the baseplate sits flush on the tibial surface, along the sagittal cut of the tibial eminence, and positioned translationally from anterior to posterior so the anterior surface is aligned with the anterior cortex (TECH FIG 4A).
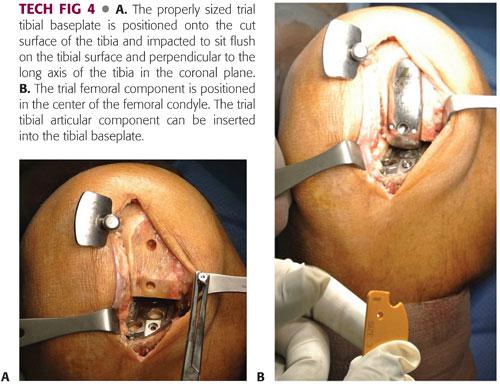
The trial femoral component is impacted in place (TECH FIG 4B).
A trial tibial articular insert is snapped into the tibial baseplate.
With the trial bearing in place, the knee is manipulated through a full range of motion to demonstrate stability of the joint, balanced flexion and extension gaps, 1 to 2 mm of laxity, restoration of alignment (without overcorrection), security of the bearing (in the case of mobile bearings), acceptable contact of the femoral component on the tibial component, and absence of patellar impingement on the leading edge of the prosthesis.
Once trial reduction is complete, all trial components are extracted.
 Implanting the Final Components
Implanting the Final Components
The femoral and tibial bone surfaces are irrigated using pulsatile lavage and then dried.
Polymethylmethacrylate is used for fixation of the final components.
The knee is flexed and the tibia externally rotated to optimize exposure of the proximal tibia for cementing.
A very thin layer of cement is pressurized into the tibia and a small amount is applied to the undersurface of the tibial component. The tibial component is pressed down and impacted into place, first posteriorly and then anteriorly, so that excess cement is preferentially extruded anteriorly. Excess extruded cement is cleared from the margin of the metal tibial tray (TECH FIG 5A).
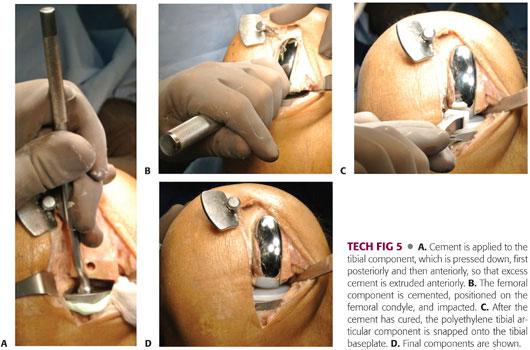
The dried femoral surface is covered with cement, and a small amount is applied to the undersurface of the femoral component. It is impacted into place (TECH FIG 5B), and excess extruded cement is removed from the margin of the femoral component.
The joint space is irrigated and the tibial tray dried before snapping the polyethylene tibial articular component into the tibial baseplate (TECH FIG 5C,D).
The knee is maintained in approximately 30 degrees of flexion to concentrically load the components while the cement hardens. A 1- to 2-mm spacer can help pressurize the components while the cement cures.
Keeping the knee fully extended during cement curing may cause the components to lift up slightly posteriorly and should be avoided.
After the cement has cured, the joint is reassessed to ensure that no visible cement is present beyond the margins of the implants. The knee is irrigated to ensure that no bone or cement particles remain.
The knee is again evaluated through a full range of motion to demonstrate stability of the joint, security of the bearing (if a mobile bearing is used), and absence of impingement.
Routine closure of the wound follows.
 Robotic-Assisted Unicondylar Knee Arthroplasty
Robotic-Assisted Unicondylar Knee Arthroplasty
Robotic assistance has been shown to enhance the accuracy of bone preparation, implant component alignment, and soft tissue balance in UKA.13,15,21,25,36,37,26
It has yet to be determined whether this improved accuracy translates to improved clinical performance or longevity of the UKA implant; however, midterm data have shown a revision rate at a minimum of 2-year follow-up of less than 1%.25
Depending on the system used, a preoperative CT scan may be needed for preoperative mapping.
Newer generation, image-free robotic technology, Navio Precision Freehand Sculpting system (NavioPFS, Blue Belt Technologies, Inc., Plymouth, MN), allows accurate bone preparation and soft tissue balancing without the need for a preoperative CT scan (TECH FIG 6A,B).
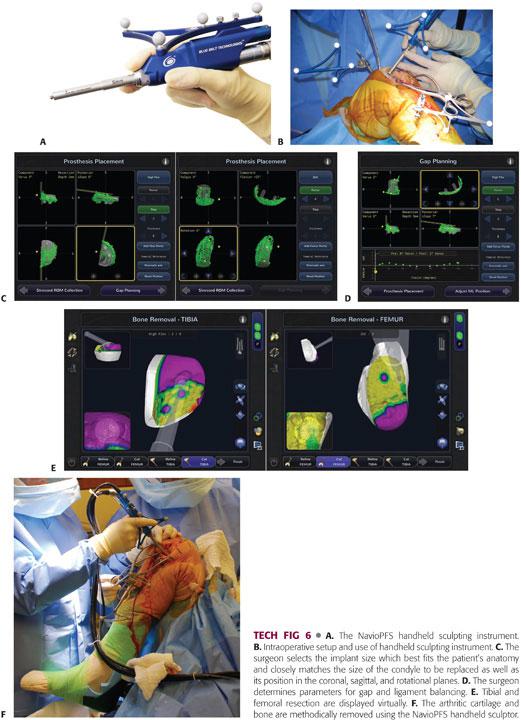
Implant planning, development of the cutting zone, and gap balancing takes place entirely intraoperatively through accurate registration and mapping of anatomic landmarks and determination of knee kinematics.
Intraoperative data are used by the system’s software algorithms to determine the coronal, sagittal, and axial bone axes and morphology.
The surgeon selects the implant size that best fits the patient’s anatomy and closely matches the size of the condyle to be replaced as well as its position in the coronal, sagittal, and rotational planes (TECH FIG 6C).
Subsequent steps are directed at determining gap and ligament balance after virtual implant positioning, removal of osteophytes, and stressing of the ligaments and soft tissues (TECH FIG 6D). Adjustments in implant position and size can be made to optimize soft tissue balance, component tracking, and position before beginning bone preparation.
After planning for size, position, alignment, bone volume, and gap balancing, the arthritic cartilage and bone are methodically removed using the NavioPFS handheld sculptor (TECH FIG 6E,F).
Unlike first-generation technologies that provided haptic constraint via a robotic arm, this newer system works with a combination of speed and exposure control safeguards applied through a lightweight, handheld, surgeon-driven, semiautonomous robotic sculpting tool.
Once the desired bone has been resected, the robotic burr either retracts into the guard when on exposure mode or stops spinning if in speed mode.
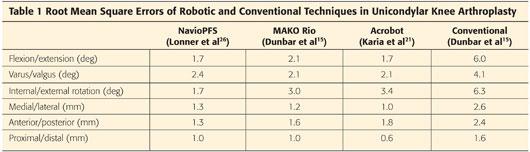
Despite relying entirely on intraoperative surface registration and mapping, the NavioPFS provides accuracy equivalent to earlier robotic devices (Table 1).26
PEARLS AND PITFALLS | |
Lengthen the incision as necessary. |
|
| |
Avoid overcorrection of the lower extremity alignment. |
|
Avoid “overstuffing” the joint space. |
|
Avoid anterior placement of the femoral component. |
|
Preserve bone stock. |
|
POSTOPERATIVE CARE
 For most patients, surgery is performed on an outpatient basis.
For most patients, surgery is performed on an outpatient basis.
 Prophylactic intravenous antibiotics are administered.
Prophylactic intravenous antibiotics are administered.
 Standard venous thromboembolism prophylaxis is implemented.
Standard venous thromboembolism prophylaxis is implemented.
 Pain management is optimized to facilitate active and accelerated rehabilitation.
Pain management is optimized to facilitate active and accelerated rehabilitation.
 Immediately following surgery, patients may weight bear as tolerated and initiate physical therapy with a focus on active-assisted muscle strengthening and range-of-motion exercises.
Immediately following surgery, patients may weight bear as tolerated and initiate physical therapy with a focus on active-assisted muscle strengthening and range-of-motion exercises.
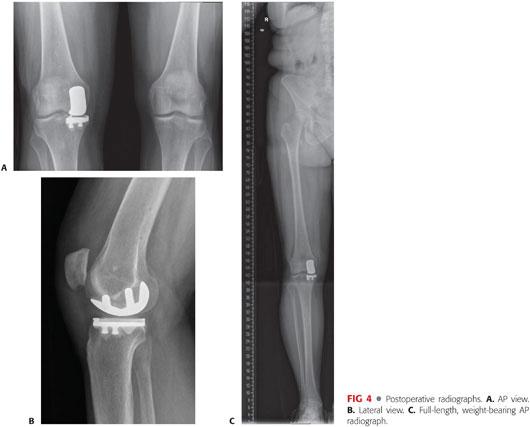
 Routine follow-up in the office is arranged for initial postoperative care and radiographic assessment of component and limb alignment (FIG 4A–C).
Routine follow-up in the office is arranged for initial postoperative care and radiographic assessment of component and limb alignment (FIG 4A–C).
OUTCOMES
 UKA is a predictable surgical option, and its potential advantages over TKA in properly selected patients include the following33:
UKA is a predictable surgical option, and its potential advantages over TKA in properly selected patients include the following33:
More conservative surgical procedure
• Less extensive surgical approach and dissection
• Preservation of native anatomy and bone stock
• Decreased blood loss
• Fewer complications
Improved rehabilitation
• Increased postoperative range of motion (FIG 5)
• Decreased rate of manipulation under anesthesia
• Preserved kinematics and proprioception
• Improved gait
 Improved logistics
Improved logistics
Shorter hospital stays and often performed in the outpatient setting
More commonly discharged to home
Decreased readmission rate
Decreased cost
 Patients report better satisfaction and fewer residual symptoms with UKA versus TKA in comparison studies.14
Patients report better satisfaction and fewer residual symptoms with UKA versus TKA in comparison studies.14
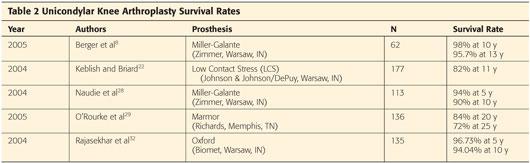
 The 10-year survival rate of UKA is greater than 90% (Table 2).
The 10-year survival rate of UKA is greater than 90% (Table 2).
COMPLICATIONS
 Tibial and femoral component and/or limb malalignment is poorly tolerated in UKA and can jeopardize long-term survival of the implant due to increased polyethylene wear, disease progression to the adjacent compartments, and component loosening.12
Tibial and femoral component and/or limb malalignment is poorly tolerated in UKA and can jeopardize long-term survival of the implant due to increased polyethylene wear, disease progression to the adjacent compartments, and component loosening.12
Polyethylene wear is the major cause of osteolysis and is minimized by the following2,3:
• Use of a polyethylene thickness greater than 6 mm
• Use of highly cross-linked, oxidatively stable polyethylene
• Appropriate component position
Adjacent compartment degeneration is caused by the following33:
• Progression of disease
• Component impingement (patellofemoral)
• Overcorrection of the mechanical axis of the lower extremity
Aseptic loosening is related to the following12:
• Poor cement technique
• Component malalignment
• Excessive tibial slope
 Other potential complications include the following:
Other potential complications include the following:
Infection
Fracture
Polyethylene dislocation (mobile-bearing UKA)
Venous thromboembolism
REFERENCES
1. Adouni M, Shirazi-Adl A. Evaluation of knee joint muscle forces and tissue stresses-strains during gait in severe OA versus normal subjects. J Orthop Res 2014;32(1):69–78.
2. Argenson JA, O’Connor JJ. Polyethylene wear and meniscal knee replacement. J Bone Joint Surg Br 1992;74:228–232.
3. Argenson JA, Parratte S. The unicompartmental knee: design and technique considerations in minimizing wear. Clin Orthop Rel Res 2006;452:137–142.
4. Argenson JA, Parratte S, Bertani A, et al. The new arthritic patient and arthroplasty treatment options. J Bone Joint Surg Am 2009;91(suppl 5): 43–48.
5. Arno S, Maffei D, Walker PS, et al. Retrospective analysis of total knee arthroplasty cases for visual, histological, and clinical eligibility of unicompartmental knee arthroplasties. J Arthroplasty 2011;26(8):1396–1403.
6. Berend KR, Lombardi AV Jr, Adams JB. Obesity, young age, patellofemoral disease, and anterior knee pain: identifying the unicondylar arthroplasty patient in the United States. Orthopaedics 2007; 30(5 suppl):19–23.
7. Berend KR, Lombardi AV Jr, Mallory TH, et al. Early failure of minimally invasive unicompartmental knee arthroplasty is associated with obesity. Clin Orthop Relat Res 2005;440:60–66.
8. Berger RA, Menegini RM, Jacobs JJ, et al. Results of unicompartmental knee arthroplasty at a minimum of ten years of follow-up. J Bone Joint Surg Am 2005;87:999–1006.
9. Billante MJ, Diduch DR. Knee replacement in aging athletes. In: DeLee JC, Drez D, Miller MD, eds. DeLee and Drez’s Orthopaedic Sports Medicine, ed 3. Philadelphia: W.B. Saunders, 2009: chap 23.
10. Borus T, Thornhill T. Unicompartmental knee arthroplasty. J Am Acad Orthop Surg 2008;16:9–18.
11. Cavaignac E, Lafontan V, Reina N, et al. Obesity has no adverse effect on the outcome of unicompartmental knee replacement at a minimum follow-up of seven years. Bone Joint J 2013:95-B(8):1064–1068.
12. Collier MB, Eickmann TH, Sukezaki F, et al. Patient, implant, and alignment factors associated with revision of medial compartment unicondylar arthroplasty. J Arthroplasty 2006;21(6 suppl 2):108–115.
13. Conditt MA, Roche MW. Minimally invasive robotic-arm-guided unicompartmental knee arthroplasty. J Bone Joint Surg Am 2009;9(1 suppl 1):63–68.
14. Dalury DF, Fisher DA, Adams MJ, et al. Unicompartmental knee arthroplasty compares favorably to total knee arthroplasty in the same patient. Orthopaedics 2009;32(4).
15. Dunbar NJ, Roche MW, Park BH, et al. Accuracy of dynamic tactile-guided unicompartmental knee arthroplasty. J Arthroplasty 2012; 27(5):803–808.
16. Fitz W. Unicompartmental knee arthroplasty with use of novel patient-specific resurfacing implants and personalized jigs. J Bone Joint Surg Am 2009;9(1 suppl 1):69–76.
17. Goodfellow JW, O’Connor JJ. The anterior cruciate ligament in knee arthroplasty. Clin Orthop Relat Res 1992;276:245–252.
18. Hernigou P, Deschamps G. Posterior slope of the tibial implant and the outcome of unicompartmental knee arthroplasty. J Bone Joint Surg Am 2004;86:506–511.
19. Hsu RW, Himeno S, Coventry MB, et al. Normal axial alignment of the lower extremity and load-bearing distribution at the knee. Clin Orthop Relat Res 1990;255:215–227.
20. Iorio R, Healy WL. Unicompartmental arthritis of the knee. J Bone Joint Surg Am 2003;85:1351–1364.
21. Karia M, Masjedi M, Andrews B, et al. Robotic assistance enables inexperienced surgeons to perform unicompartmental knee arthroplasties on dry bone models with accuracy superior to conventional methods. Adv Orthop 2013;2013:481039.
22. Keblish PA, Briard JL. Mobile-bearing unicompartmental knee arthroplasty: a 2-center study with an 11-year (mean) follow-up. J Arthroplasty 2004;19(7 suppl 2):87–94.
23. Kozinn SC, Scott R. Unicondylar knee arthroplasty. J Bone Joint Surg Am 1989;71:145–150.
24. Laskin RS. Unicompartmental knee replacement: some unanswered questions. Clin Orthop Relat Res 2001;392:267–271.
25. Lonner JH, John TK, Conditt MA. Robotic arm-assisted UKA improves tibial component alignment: a pilot study. Clin Orthop Relat Res 2010;468:141–146.
26. Lonner JH, Smith JR, Picard F, et al. High degree of accuracy of a novel image-free handheld robot for unicondylar knee arthroplasty in a cadaveric study. Clin Orthop Relat Res 2015;473(1):206–212.
27. Mihalko WM. Arthroplasty of the knee. In: Canale ST, Beaty JH, eds. Campbell’s Operative Orthopaedics, Vol 1, ed 12. Philadelphia: Mosby, 2012:chap 7.
28. Naudie D, Guerin J, Parker DA, et al. Medial unicompartmental knee arthroplasty with the Miller-Galante prosthesis. J Bone Joint Surg Am 2004;86:1931–1935.
29. O’Rourke MR, Gardner JJ, Callaghan JJ, et al. Unicompartmental knee replacement: a minimum twenty-one-year followup, end-result study. Clin Orthop Relat Res 2005;440:27–37.
30. Pandit H, Jenkins C, Gill HS, et al. Unnecessary contraindications for mobile-bearing unicompartmental knee replacement. J Bone Joint Surg Br 2011;93(5):622–628.
31. Pennington DW, Swienckowski JJ, Lutes WB, et al. Lateral unicompartmental knee arthroplasty: survivorship and technical considerations at an average follow-up of 12.4 years. J Arthroplasty 2006;21:13–17.
32. Rajasekhar C, Das S, Smith A. Unicompartmental knee arthroplasty 2- to 12-year results in a community hospital. J Bone Joint Surg Br 2004;86:983–985.
33. Ritter MA, Faris PM, Thong AE, et al. Intra-operative findings in varus osteoarthritis of the knee: an analysis of pre-operative alignment in potential candidates for unicompartmental arthroplasty. J Bone Joint Surg Br 2004;86(1):43–47.
34. Scott RD, Santore RF. Unicondylar unicompartmental replacement for osteoarthritis of the knee. J Bone Joint Surg Am 1981;63(4):536–544.
35. Servien E, Merini A, Lustig S, et al. Lateral unicompartmental knee replacement: current concepts and future directions. Knee Surg Sports Traumatol Arthrosc 2013;21:2501–2508.
36. Sisto DJ, Blazina ME, Heskiaoff D, et al. Unicompartment arthroplasty for osteoarthritis of the knee. Clin Orthop Relat Res 1993;286: 149–153.
37. Smith JR, Picard F, Rowe PJ, et al. The accuracy of a robotically-controlled freehand sculpting tool for unicondylar knee arthroplasty. J Bone Joint Surg Br 2013;95B(suppl):68.
38. Stern SH, Becker MW, Insall JN. Unicondylar knee arthroplasty: an evaluation of selection criteria. Clin Orthop Relat Res 1993;286: 143–148.
39. White SH, Ludkowski PF, Goodfellow JW. Anteromedial osteoarthritis of the knee. J Bone Joint Surg Br 1991;73:582–586.
< div class='tao-gold-member'>









