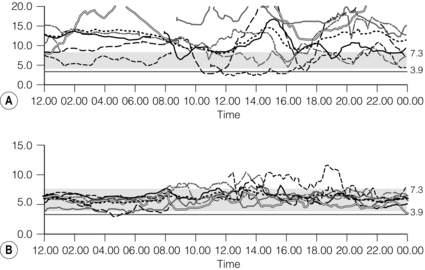
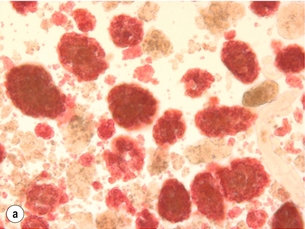
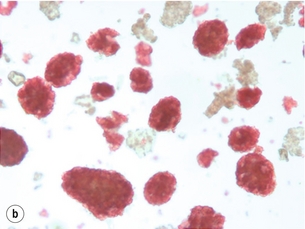
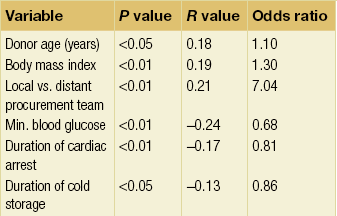
10 The discovery of insulin by Banting and Best in the early part of the 20th century saw type I diabetes become a treatable chronic illness rather than a rapidly fatal diagnosis. Since then, patients with diabetes have been able to lead relatively normal lives thanks to ongoing refinements in insulin therapy. Despite this, many patients will suffer from the secondary side-effects of diabetes including retinopathy, neuropathy, nephropathy and premature cardiovascular disease. The Diabetes Control and Complications Study Group Trial demonstrated that the incidence of these complications can be reduced by tight glycaemic control but the risk of severe hypoglycaemic reactions is increased.1 Follow-up of this cohort of patients confirmed that these benefits were long lasting even if patients were unable to maintain tight control in the long term.2 Patients with type I diabetes and end-stage diabetic nephropathy may benefit from simultaneous pancreas and kidney transplantation (SPK). This not only removes the need for dialysis in these patients, but also results in normoglycaemia without the need for insulin in over 85% of patients.3 Unfortunately, the morbidity and mortality associated with SPK transplantation mean that only a small number of patients are fit enough to undergo SPK transplantation and in addition many more patients have labile diabetes without concurrent renal failure.4 Many of the complications of whole pancreas transplantation are associated with the exocrine portion of the pancreas (pancreatitis, pancreatic fistula). The islets of Langerhans account for only 1% of the mass of the pancreas but contain the beta and alpha cells responsible for insulin and glucagon production necessary for glycaemic control. The concept of extracting and transplanting islets is not new and was initially attempted in 1893 by P. Williams in Bristol. He transplanted a fragmented sheep’s pancreas subcutaneously into a 15-year-old boy dying of ketoacidosis.5 This early xenograft was, not surprisingly, unsuccessful but predated the discovery of insulin by nearly 30 years. The era of experimental islet research began in 1911, when Bensley stained islets within the guinea-pig pancreas using a number of dyes, and was able to pick free the occasional islet for morphological study.6 Mass isolation of large numbers of viable islets from the human pancreas has proven to be a challenge ever since. The average adult human pancreas weighs 70 g, contains an average of 1–2 million islets of average diameter 157 μm, constituting between 0.8% and 3.8% of the total mass of the gland.7 It was almost 100 years after the work of Bensley that Scharp et al. reported insulin independence after islet transplantation; however, even this was short lived and difficult to reproduce.8,9 This remarkable outcome was achieved by transplanting at least two islet preparations from different donors and using a novel steroid-free immunosuppression regimen of tacrolimus, sirolimus and induction with daclizumab. This regimen has been termed ‘the Edmonton protocol’ and many units worldwide have attempted to replicate these outcomes, with variable success.11,12 In the aftermath of the Edmonton protocol, islet transplantation is considered in many countries as ‘standard of care’ for a select group of patients with type I diabetes and is funded through the healthcare system in Canada and the NHS in the UK. The results of combined islet and kidney transplantation now match those of islet alone,13,14 and recent data suggest that islets transplanted with a kidney may prolong the patient and kidney graft survival and protect against diabetic vascular complications.15,16 There are two principal indications for islet transplantation: 1. Severely impaired awareness of hypoglycaemia (IAH) despite optimum insulin therapy. IAH occurs in 20–25% of patients with type I diabetes and is potentially life threatening. These patients have a defective counter-regulatory hormonal response to hypoglycaemia, are unable to identify low blood sugars and therefore institute corrective measures.4 Defective recognition of hypoglycaemia increases the risk of severe hypoglycaemic episodes that can result in coma and death. The impact on quality of life for these patients is substantial, and social activities and employment can be severely restricted; indeed, in the UK, patents with IAH cannot hold a UK driving licence. 2. Patients with type I diabetes and a functioning kidney allograft who are unable to maintain their HbA1c below 7%. In this patient group it is not necessary to demonstrate IAH as they are already taking immunosuppression and it has been shown that the improved glycaemic control after islet transplantation in this setting is associated with a reduction in long-term diabetic complications. Impaired awareness of hypoglycaemia can be assessed by patient history and by asking the patient to keep a diary of insulin usage, dietary intake and hypoglycaemic events, in particular those events requiring assistance from relatives or those requiring hospitalisation. The use of a continuous glucose monitoring sensor (CGMS) can be very useful in assessing daily glucose profiles pre- and post-islet transplantation (Fig. 10.1).17 Scoring systems such as the Gold or Clark scores allow numerical documentation of the degree of IAH. The Gold score asks the question ‘do you know when your hypos are commencing?’ and the patient completes a linear scale from 1 to 7 (always aware to never aware). A score of 4 or above suggests IAH. The Clark method asks eight questions to document the patient’s exposure and responses to moderate and severe hypoglycaemia, and again a score of 4 or more suggests IAH.4 Ryan et al. have described a composite HYPO score based on 4 weeks of glucose values.18 They suggest that this provides a more objective assessment of the metabolic instability of an individual patient and allows pre- and post-transplant comparison. Patients should be assessed by a multidisciplinary team consisting of a diabetologist, transplant surgeon, dietician and a diabetes nurse specialist. This will ensure that their current insulin regimen and dietary compliance are optimum and that the patient is fully informed about the likely outcome of islet transplantation and the risks involved, principally post-transplant immunosuppression. Donor factors contributing to successful islet isolation have been documented by Lakey et al. (Table 10.1).19 This paper suggests that pancreata from older donors with a higher body mass index (BMI) should result in a significantly higher islet yield. O’Gorman et al. have suggested a scoring system from 1 to 100 to give a numerical assessment of the likelihood of successful isolation from a specific donor pancreas.20 These studies, however, only predict successful isolation and do not take into consideration data that suggest that islets isolated from younger donors are functionally better.21 In the UK a sharing scheme was introduced in December 2010 where patients for SPK transplantation and islet transplantation are placed on a common waiting list and pancreata offered on a named patient basis. Multiple donor and recipient factors are taken into consideration, allowing islet and whole pancreas recipients equal access to suitable organs. Table 10.1 Donor-related variables predicting isolation success Reproduced from Lakey JR, Warnock GL, Rajotte RV et al. Variables in organ donors that affect the recovery of human islets of Langerhans. Transplantation 1996; 61(7):1047–53. With permission from Lippincott, Williams & Wilkins. Most of the outcome data on islet transplantation are based on organs from brain dead donors (DBD); however, there is growing evidence that pancreata from donation after circulatory death (DCD) can produce transplantable preps and good outcomes. Most of the data on DCD islet transplantation are from the Kyoto group and although long-term graft survival is obtained, insulin independence is less common.22–26 It is critical that the pancreas for islet isolation is retrieved with the same care as that for whole pancreas transplantation and that the cold ischaemic time is minimised, ideally to under 8 hours.27 Pancreata should be transported rapidly and the staff in the isolation laboratory should be ready to begin the isolation immediately. It has been demonstrated that suspending the explanted pancreas in a bilayer of oxygenated perfluorocarbon (PFC) and University of Wisconsin (UW) solution during or after transport allows satisfactory islet preparations to be obtained from suboptimal pancreases and may even increase yields from pancreases with long ischaemia times.28,29 PFC-based preservation may also help expand the donor pool by improving islet isolation from DCD pancreases and older donors.30 The semi-automated process for islet isolation that is used in most labs was described by Ricordi et al. in 1989.31 This involves digestion of the pancreas using a combination of collagenase enzyme and mechanical dissociation of the pancreas in the Ricordi chamber (Fig. 10.2). A number of new enzyme blends have been developed for human isolation, including collagenase NB1 (Serva), Liberase MTF (Roche) and C1 collagenase HA (Vitacyte). Each of these differs slightly in the enzyme blend and manufacturing process but promises to deliver more consistent, better quality islet yields. The prep is then purified on a continuous density gradient using a COBE 2991 cell separator, resulting in a packed cell volume of only 1–2 mL (Fig. 10.3).32 Although unpurified preps can be used (particularly in autotransplants), the risk of portal vein thrombosis, portal hypertension and disseminated intravascular coagulation (DIC) is increased.33–36 After isolation and purification, it is now standard practice to place the islet prep in culture for 12–48 hours. There are compelling data that this does not adversely affect islet graft function and does in fact increase purity of the prep.37 Extended culture up to 48 hours can also be used to assess the viability of an islet graft, particularly after DCD isolation. The number of islets in the prep is documented in terms of islet equivalents (IEQ). This counting method adjusts for the fact that islets vary greatly in size and cell viability stains such as fluorescenediacetate/propidium iodide and SytoGreen/ethidium bromide are used to determine the viable beta-cell mass.38 The minimum release criteria in the UK for an islet preparation are: It is accepted that these criteria are subjective and open to observer variation and error. Some assessment of the ‘quality’ of the prep should also be made. Experienced islet lab staff can comment on the morphology of the cells, the integrity of the islets and whether or not there is evidence of central necrosis within the islets. Islet oxygen consumption rate and beta-cell ATP content show good correlation between product testing and in vivo islet function in animal studies, and may be useful in the future but are time consuming and expensive. The Minnesota group has demonstrated good correlation between marginal mass islet transplants in diabetic nude mice and outcome of human islet transplants from the same donor.39
Islet transplantation
Introduction
Patient selection and assessment
Islet isolation
Islet transplantation
Only gold members can continue reading. Log In or Register to continue

Full access? Get Clinical Tree