Internal Mammary Sentinel Lymph Node Biopsy
Hiram S. Cody III
Virgilio Sacchini
Introduction
The predominant lymphatic drainage of the breast is to the axillary nodes but the internal mammary nodes (IMNs) have long been recognized as an alternate pathway (1,2).
The historic rationale for “extended radical mastectomy” (ERM) (3), an operation that combined a full-thickness resection of the parasternal chest wall and IMN with a classic radical mastectomy (RM), was the observation by Urban (4) in 1951 of a very high rate of parasternal chest wall recurrence following RM in patients with inner quadrant breast cancers.
The goal of ERM was to reduce the rate of local recurrence and, by improving local control, to improve survival. This goal was never met: Veronesi’s randomized trial comparing ERM with RM demonstrates a 1.1% to 3.5% reduction in the 10-year rate of parasternal chest wall recurrence (5) but no difference in survival at 30 years’ follow-up (6).
The published experience with ERM (comprising 4,172 patients in 7 studies) (7) is instructive and relevant to the current era of sentinel lymph node (SLN) biopsy. IMN metastases
were present in 19% to 33% of all patients,
were more frequent in axillary node-positive (29%–52%) than node-negative (4%–18%) patients,
were equally frequent for central/medial versus lateral tumors if the axillary nodes were negative (8%–10% vs. 3%–13%), and
were more frequent for medial/central tumors if the axillary nodes were positive (36%–49% vs. 22%–26%).
The principal reason for surgical staging of lymph nodes in breast cancer is prognostication. The prognosis of patients with metastases limited to the IMN or to the axillary nodes is comparable and is intermediate between that of patients with negative nodes and those with both IMN and axillary metastases (7,8,9). The identification of IMN metastases is, therefore, of particular importance for patients who would not
otherwise be candidates for systemic adjuvant therapy, that is, those with negative axillary nodes and tumors smaller than 1 cm. If one assumes that this subset (perhaps 5% of all patients) received systemic therapy, then less than 1% of all patients would experience a survival benefit.
A secondary goal of lymph node surgery is local control. Current treatment protocols for stage I to II breast cancer do not include any IMN treatment, and yet local recurrence in the IMN or parasternal area occurs in less than 1% of patients treated by either mastectomy (10) or breast conservation therapy (BCT) (11). The recent Oxford Overview (12), a landmark in the history of breast cancer treatment, clearly demonstrates a relationship between local control and survival, but only for those treatment strategies in which local recurrence was reduced by more than 10%. Local recurrence in untreated IMN is already rare, and for this reason, it is inconceivable that any further reductions in the rate of local recurrence could affect survival.
SLN biopsy, in which radioisotope and blue dye are used to map the first few lymph nodes draining the breast, has now replaced axillary dissection (ALND) as standard care for patients with operable breast cancer (13). SLN biopsy has also revived debate over the significance of nonaxillary lymphatic drainage, particularly to the IMN. In a remarkable series of 700 SLN biopsy procedures (done with meticulous lymphatic mapping by intratumoral injection), Estourgie et al.’s (14) reported results that largely recapitulate those from the era of ERM: by preoperative lymphoscintigraphy (LSG), 95% of patients drained to the axilla and 22% to the IMN. Among those with IMN drainage, nodes were seen most frequently in the third (36%), second (27%), and fourth (24%) interspaces.
IM-SLN Biopsy
The above-mentioned data suggest that the benefit of IM-SLN biopsy will accrue to very few patients. Nevertheless, there are several clear indications for the procedure:
Preoperative LSG showing drainage only to the IMN. IMN drainage is almost always accompanied by axillary drainage, but for the few patients who map exclusively to the IMN, IM-SLN biopsy makes sense and allows the surgeon to avoid unnecessary exploration of the axilla.
Preoperative LSG showing drainage to IMN and axillary nodes. IM-SLN biopsy is reasonable in this setting if (a) the axillary SLN is benign on intraoperative examination and (b) the patient is not already a candidate for adjuvant chemotherapy on the basis of other criteria. While IM-SLN biopsy makes sense for any patient in whom a positive result would alter the plan for systemic therapy, this decision is increasingly based on factors other than lymph node status, among them ER/PR/her2 status, lymphovascular invasion, and (increasingly) gene expression profiling.
Preoperative imaging (computed tomography [CT], magnetic resonance imaging, positron emission tomography) with evidence of IMN involvement. Grossly enlarged IMN are usually amenable to CT-guided core biopsy and are thus a debatable indication for IM-SLN biopsy. As noted earlier, most patients with visible IMN metastases will be candidates for chemotherapy on the basis of other criteria. There are no data to suggest that surgical excision of grossly involved IMN (and specifically IM-SLN biopsy) will improve local control beyond that achieved by chemotherapy and radiotherapy (RT).
“Reoperative” SLN biopsy with drainage to IMN. SLN biopsy is feasible in patients who have had prior axillary surgery (either SLN biopsy or ALND) for breast cancer and present with local recurrence (15).
Lymphatic mapping in the reoperative setting is particularly useful since the prior surgery may have altered the lymphatic drainage of the breast unpredictably.
We have observed nonaxillary drainage (most often to the IMN) in 30% of reoperative SLN biopsies vs. 6% of our “first-time” procedures (16).
Ipsilateral recurrence in the conserved breast occurs in approximately 5% to 10% of all patients, and these are the patients for whom IM-SLN biopsy may ultimately prove to be most useful.
The success of SLN biopsy is maximized by a combination of radioisotope and blue dye mapping (17), and isotope is crucial for IM-SLN biopsy. 99mTechnetium is complexed to a variety of carrier particles (sulfur colloid in the U.S., colloidal albumin in Europe, and antimony in Australia) and injected into the breast either the day before or the morning of surgery; we have observed identical results with day-before or same-day isotope injection (18).
The dermal lymphatics of the breast drain almost exclusively to the axilla and the deeper breast lymphatics drain to the IMN and axilla (19).
The success of axillary SLN biopsy is maximized by superficial injections of isotope (intradermal, subdermal, or subareolar) and intraparenchymal/peritumoral injection is somewhat less successful. These results come from many observational studies and have been confirmed in a randomized trial (20).
The success of IM-SLN biopsy is maximized by deep injections of isotope (intraparenchymal/peritumoral/intratumoral).
Preoperative LSG is of arguable benefit if the goal at surgery is to identify axillary SLN (a handheld gamma probe is more sensitive that a full-field-of-view gamma camera) but is absolutely essential to identify nonaxillary patterns of lymphatic drainage, and particularly IM-SLN.
In summary, for those clinical settings where IM-SLN biopsy is a priority, a combination of superficial and deep injections of isotope will maximize success overall, and preoperative LSG is mandatory.
Relevant Anatomy
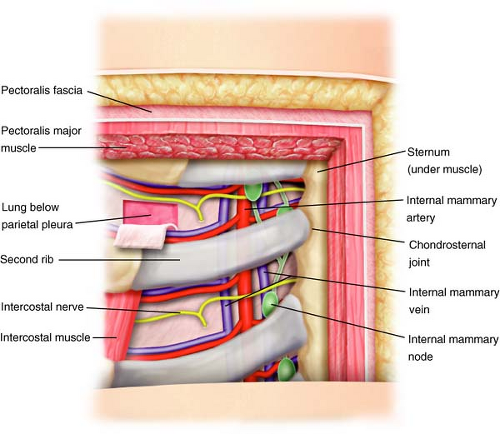
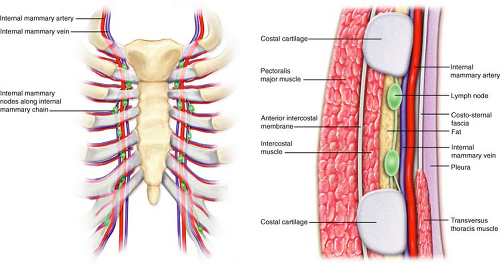
The IMN chain is confined just anterior to the extrapleural chest space approximately 2 to 3 cm lateral to the sternal border. Two IM veins parallel the IM artery, one medial and one lateral to it (Fig. 11.1).
The IM artery runs through this same space along the lateral sternal border approximately 10 mm from the border at the first intercostal space increasing slightly to 20 mm at the sixth space.
From the IM artery, derive the anterior intercostal arteries, two for each intercostal space, running between the two intercostal muscles, one inferior and the other superior, both anastomosed with the posterior intercostal artery (Fig. 11.1).
Injection of Blue Dye
In the operating room, with the patient either sedated (for breast conserving surgeries) or under general anesthesia (for mastectomy), the chest is prepped and draped in the usual sterile manner.
Next, a subdermal injection of 1 to 5 cc of isosulfan blue dye is given in one of three ways:
directly over the tumor
just cephalad to the prior excision scar
in the subareolar location.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree