Key points
- •
Must use medical grade product that is FDA approved for injection into the human body.
- •
Indicated but ‘off-label’ for HIV- or age-related lipoatrophy, as well as correction of nasolabial folds, marionette lines, malar cheek and lip enhancement, and acne scars.
- •
Usage of the microdroplet technique at multiple treatment sessions spaced at least 1 month apart.
- •
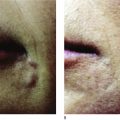
Nodule formation is extremely rare when performed correctly.
- •
Future studies are needed to improve general acceptance of the product as safe.
History of silicone
Injection of Dow Corning’s wartime non-human grade silicone oil into the human body for soft tissue augmentation began in the USA in the 1950s after years of off-label usage overseas. It was noted, however, that the implants made of large quantities of the oil could migrate along tissue planes to distant sites, so many tissue irritants were added to the oil to try to stimulate a fibrous reaction at the implant site to hold it in place. This adulterated product caused many granulomatous reactions to the implants.
In the 1960s, Dow Corning introduced DC 360 Medical Fluid, a relatively purified 350 centastoke non-sterile non-human grade silicone oil intended to coat medical supplies like needles and syringes. Unregulated non-standardized human injections for soft tissue augmentation again continued without appropriate protocols which led to frequent reports of inflammatory reactions, silicone oil migration and to the FDA’s regulation of the DC 360 Medical Fluid.
In 1965, a poorly controlled FDA approved study on 1300 patients of soft tissue augmentation with the new sterilized DC MDX 4-4011 was initiated by Dow Corning. Protocols were not rigorously followed but outlined small aliquots of silicone injected over several sessions. One patient reported a negative outcome and was later found to have had a large volume injection and tissue migration. The study was halted secondary to inability to prevent product misuse, however, continued injection of non-investigational and non-injectable-grade products continued. In 1975, Nevada passed a statewide law banning the use of injectable silicone.
In 1978, a 128 patient Dow Corning study was FDA approved to study MDX 4-4011 for correction of severe atrophic deformities. Unfortunately, many variables including injection volumes, techniques and number of treatments were still not standardized. Even without rigorous protocols, 98% of cases reported good to excellent treatment outcomes. Thirty-six device related complications were reported in 23 of these good to excellent outcome cases and many described transient normal sequelae of injection. However, two patients had severe complications requiring surgery.
The 1980s brought continued usage of non-standardized silicone that was mislabeled ‘medical-grade’ and contained varying levels of adulterants and impurities. This poor reputation spilled over into the 1990s when, in 1992, the FDA restricted use of all silicone products because of possible toxicity of silicone breast implants. However, 1994 was a turning point for liquid injectable silicone (LIS) when the FDA approved Bausch & Lomb’s Adato® SiL-oL 5000 for treatment of complicated retinal detachment and soon after approved Alcon’s less viscous LIS, Silikon™ 1000, for intraocular use. In 1997, having highly purified and standardized LIS available, the FDA approved the use of these products ‘off-label’ for soft tissue augmentation based on the needs of the patient as long as the product is not marketed for that purpose. Physicians must still check with their liability carriers for coverage of LIS usage off-label.
Currently, there are well-designed clinical trials with rigorous protocols for the 1000-centistoke oil SilSkin™ created specifically for soft tissue augmentation of nasolabial folds, marionette lines, and malar depressions as well as for the treatment of HIV lipoatrophy. These trials limit the LIS volume injected per session as well as the number of total injections permitted and include a follow-up period. These trials will hopefully provide objective data on outcomes of using medical grade silicone in standardized small volumes.
Properties of silicone
The term ‘silicone’ was coined by a British chemist in the early 1900s to describe a large family of synthetic polymers contained the element silicon. These polymers have varying viscosities and can exist as solids, gels, and liquids. LIS consists of the compound siloxane, which is a acronym for elemental silicone conjugated with oxygen and methane to form dimethylpolysiloxane fluid. The viscosity of silicone liquid is measured in centistokes (cS), with the viscosity of water at 100 cS.
In most ways, silicone appears to fulfill criteria for an ideal implantable substance. It is permanent, non-carcinogenic, minimally antigenic, and does not support bacterial growth. It can be easily heat sterilized. It is important to note that silicone injected into different anatomical sites has differing risk/benefit profiles. For example, the risk of granulomas reported after foot injections is much lower than that reported for the face. Also, the risk profile of silicone-containing breast implants, and elastomers can not be directly compared to liquid silicone.
Unfortunately, its controversial history of misuse and lack of standardization has created a stigma for the product and its usage. Hopefully, the current ongoing clinical investigations of LIS injections will provide positive data supporting future use of this product with responsible protocols utilizing only medical grade product in the microdroplet technique at the appropriate depth.
Patient selection and indications
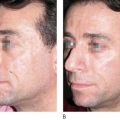
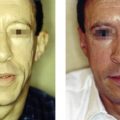
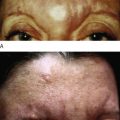
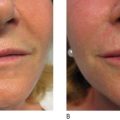
Liquid injectable silicone (LIS) is indicated for the correction of nasolabial folds and marionette lines, volume loss of the malar cheek, lip atrophy, tear trough depression, acne and other atrophic scarring, and HIV or age-related lipoatrophy. It is contraindicated for injection into the breasts or any actively inflamed site. Patients must have realistic expectations and be aware that this is not an immediate correction, but must be completed over multiple treatment sessions. They must understand that they may not see any results until the third treatment. The doctor must also be able to tell the patient that they have had enough so that they do not overcorrect. The microdroplet technique is the only one that should be used and injections should not be done more often than at 4–6 week intervals. If possible, it is always good to start with a non-permanent filler so that the patient is sure that they like the result.
Treatment should not be performed in patients with significant dental caries or chronic sinusitis secondary to possible risk of biofilms with granuloma formation. Treatment is permanent but the patient may need more treatments after a few years as they continue to age. Silicone injections are a very common treatment for diabetic ulcers, corns and other podiatric problems. These procedures have been performed successfully for many years.
Operative technique
Pre-operative preparation
Verbal and written informed consent must be obtained and include the acknowledgment that LIS is a permanent filler and is being used off-label. High-resolution pre-treatment photographs should be obtained. Patients should avoid aspirin, non-steroidal anti-inflammatory products and blood thinners for 10–14 days before treatment.
The skin is cleansed with iodine swabs and an antibacterial cleanser to remove any make-up or debris. Anesthesia can be achieved with local nerve blocks or an anesthetic cream applied topically. Usually, an anesthetic cream is enough as this filler is not very painful to inject. It is always preferable to use an anesthetic block when injecting any filler into the lips. With the patient in the sitting position, treatment areas are marked. Overhead lighting that accentuates the cosmetic defects should be used. Markings should be made with the patient in both the smiling and resting position.
Technique
Only pure, medical grade LIS approved by the FDA for injection into the human body must be used. In the USA currently, LIS injectors use Silikon 1000 (Alcon, Fort Worth, TX) as other medical grade silicones like Adato® SiL-oL 5000 proved too viscous for practical use in the skin. Patients must be aware that currently silicone oil is only FDA approved for use in the eye for severe retinal detachment. All other uses are ‘off-label’. Check with your malpractice insurance company to make sure you are covered to do this procedure. It varies state by state in the USA.
Using standard sterile technique the oil is drawn through a 16-gauge needle into a 1 cc Luer-Lok syringe immediately prior to injection. It is advisable to only fill to the 0.5 cc mark or less to maintain good plunger control. A 27 gauge needle should be used for injection now that the 30 g Max-Flo needle with a 27 g bore is no longer available. Injectors have noted that plastic hubs tend to pop off from the injection pressure used with smaller gauge needles. Some advise autoclaving and utilizing a 0.5 inch diameter rubber electrical bush from the hardware store over the barrel of the syringe to cushion the injector’s second and third fingers.
Currently, the microdroplet serial puncture technique as described by Orentreich ( ) is primarily used by all injectors. Injections must be made into the immediate subdermal plane and intradermal injection must be avoided to prevent dermal erythema, nodules, or ridging. The needle is advanced through the dermis and as it enters the subdermal plane a slight loss of resistance is felt. At this plane, microdroplets of 0.0005–0.001 ml are placed. The thumb should be removed from the plunger when pulling the needle out of the skin to avoid tracking product through the dermis. Injections should be spaced at 2–5 mm intervals. All but very experienced injectors should avoid doing a double pass over one area in one treatment session. Total volumes should be limited to an average of 0.5 cc for smaller areas like the nasolabial fold, and no more than 2 cc for larger areas of facial lipoatrophy. Injection of larger volumes of product increases the risk for tissue tracking to distant body sites.
When larger volumes of LIS are injected, the product acts more like an oil and can track along paths of least resistance to distant sites. The microdroplet placement of LIS stays in place long enough to allow a collagenous capsule to form around it, anchor it, and prevent migration. Additionally, the increased surface area of multiple microdroplets results in increased total collagen deposition and improved clinical outcome. Intervals between each small volume treatment is typically 1 month or longer to allow for encapsulation. LIS-induced collagen formation has been estimated to be approximately 50% at 4 weeks and 100% at 12 weeks. As one gets closer to optimal correction, care must be taken not to overcorrect and the treatment intervals should be extended to allow for complete collagen deposition.
Operative steps
- •
Obtain informed consent.
- •
Take pretreatment photos.
- •
Cleanse skin with iodine.
- •
Mark treatment areas in sitting position.
- •
Apply topical anesthetic cream or nerve block.
- •
Draw up oil using sterile technique.
- •
Inject with microdroplet technique.
- •
Apply ice.
- •
See patient back in 4–6 weeks.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree