(1)
University of Florida, College of Medicine, Gainesville, FL, USA
(2)
Private Practice:, Orlando, FL, USA
4.1 Viral Infections
A. HUMAN HERPESVIRUSES (HHV)
Eight types of HHVs:
HHV1 → HSV1 | HHV5 → CMV |
HHV2 → HSV2 | HHV6 |
HHV3 → VZV | HHV7 |
HHV4 → EBV | HHV8 → KSHV |
Neurotropic virus which hides in the dorsal root ganglion until reactivation
HSV-1 associated more with orolabial herpes, HSV-2 with genital herpes
Primary infections:
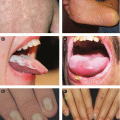
Primary herpetic gingivostomatitis: typically in children, abrupt onset with striking gingivitis (erythematous, friable gingiva), painful vesicles clustered on oral mucosa, tongue, lips and/or perioral skin → vesicles rupture, leaving small ulcers with characteristic gray base; ± pharyngitis, tonsillitis, difficult to eat and swallow, enlarged lymph nodes, fever, and anorexia
Primary genital infection: more severe and prolonged than recurrent infection, presents with constitutional symptoms and painful grouped vesicles in genitalia → progress to pustules, crusting and exquisitely tender ulcers, ± painful lymphadenopathy, cervicitis, urethritis, proctitis
Recurrent infections:
Herpes labialis: most common HSV-1 manifestation triggered by pyrexia, stress, sunburn, and/or trauma; prodrome (pain, burning, tingling) may precede eruption; grouped vesicles on erythematous base which typically evolve into pustules and then painful ulcers (often involving vermilion border)
Genital herpes: ± prodrome followed by grouped vesicles → pustules → ulceration
Other types of infections:
Herpes gladiatorum: HSV primary infection primarily noted in wrestlers and involving extramucosal sites typically over face, neck or arms
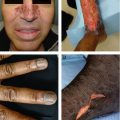
Herpetic whitlow: painful primary herpetic infection of hand (typically distal phalanx) resulting in exquisite pain and swelling of finger with characteristic vesicular lesions; more common in health care workers or caregivers
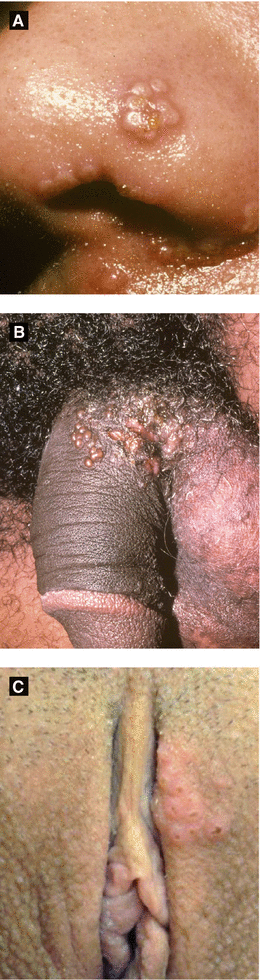
Figure 4.1:
A: Recurrent HSV infection(Courtesy of Dr. Paul Getz)B: Primary genital HSV(Courtesy of Dr. Paul Getz)C: Primary genital HSV
Eczema herpeticum (Kaposi varicelliform eruption): rare disseminated form of HSV mainly seen with atopic dermatitis (also Darier disease, Hailey-Hailey, etc); presents as monomorphic umbilicated vesiculopustules or punched out ulcerations with hemorrhagic crust; may progress to life-threatening infection (Figure 4.2A)
Herpes-associated erythema multiforme (HAEM): self-limited eruption associated with HSV infection; presents with typical concentric target plaques; begins on extremities and spreads centripetally, ± mucosal involvement
HSV encephalitis: dormant HSV in trigeminal ganglion → travels retrograde to the brain, targets temporal region of brain, 70% mortality if untreated
HSV folliculitis: rare manifestation
Chronic ulcerative HSV: presents mainly in immunocompromised patients as persistent ulcers involving perianal/buttock area, can be pustular, exophytic, or verrucous as well (Figure 4.2B)
Keratoconjunctivitis: can be primary or recurrence, latter presents typically with branching dendritic corneal ulcerations (seen with fluorescein stain), can lead to scarring and blindness (Figure 4.2C)
Diagnosis:
Tzanck smear shows multinucleated epithelial giant cells (fusion of infected keratinocytes) – does not differentiate between HSV and VZV
Viral culture or direct fluorescent antibody (DFA)
Histology shows keratinocyte edema causing ballooning degeneration and acantholysis, intranuclear inclusion bodies and dense inflammatory infiltrate ± epidermal/adnexal necrosis
Treatment: acyclovir, valacyclovir, famciclovir; if acyclovir-resistant use foscarnet or cidofovir
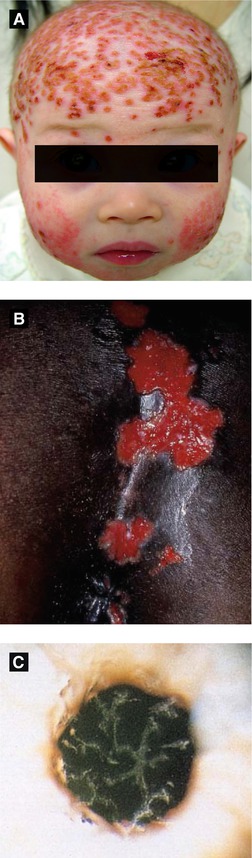
Figure 4.2:
A: Eczema herpeticum(Courtesy of Dr. Sophie M. Worobec)B: Perianal HSV ulcers(Reprint Bolognia JL, Jorizzo JL, Rapini RP. Dermatology. 2nd ed. St. Louis, MO: Mosby Elsevier; 2008.)C: HSV corneal ulcer(Reprint from Mandell G, ed. Atlas of Infectious Diseases. Philadelphia, PA: Current Medicine LLC; 2002.)
Initial infection causes varicella (chickenpox, Fig 4.3A) and following resolution, virus lies dormant in spinal dorsal root ganglion until reactivation of latent virus causing localized cutaneous eruption (herpes zoster or shingles)
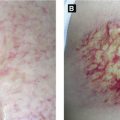
Presents with grouped, painful erythematous macules and papules along single sensory dermatome (rarely crossing midline) → vesicles/bullae → rupture forming hemorrhagic crust and become dry over 7-10 days; lesions are infectious until dry; sensory prodrome (pain, pruritus, or paresthesias) in 75% of cases and mild systemic response (headache, malaise, anorexia, etc) can precede presentation; thoracic dermatome most commonly involved
Disseminated zoster, defined as generalized eruption of more than 10-12 extradermatomal vesicles occurring 7-14 days after onset of classic dermatomal zoster, is seen in 2% of cases in general population and up to 35% in immunocompromised patients; observe these patients carefully for development of visceral involvement (liver, lung, brain) as pneumonitis and encephalitis can be life-threatening
Atypical presentation in AIDS pts: >2 dermatomes affected, may cross midline, may present with verrucous/crusted lesions
Complications: post-herpetic neuralgia (PHN), scarring, secondary bacterial infection, meningoencephalitis, Ramsay-Hunt syndrome, ocular blindness, motor paralysis
Postherpetic neuralgia (PHN): pain that continues after resolution of the eruption, typically lasting more than 30 days (some experts prefer to reserve the term for pain that lasts more than 90 days); occurs in 10-25% of cases
Ocular involvement: lesions on tip of nose signal possible ocular infection (since nasociliary nerve involved, a branch of the ophthalmic nerve)
Ramsay-Hunt syndrome: infection of geniculate ganglion → ear canal/auricle/tympanic membrane involvement with painful vesicles, facial paralysis/paresis, ipsilateral hearing loss
Treatment: antiviral best if within 48-72 h within appearance of rash (can ↓ PHN risk); of note, concomitant corticosteroid use has no effect on development/duration of PHN; IV acyclovir used in immunocompromised patient if advanced HIV, widespread skin involvement, visceral disease or transplant patients
Of note, women with varicella infection 5 days before or 2 days after delivery, can result in severe acute infection of newborn (neonatal varicella, mortality of newborn up to 30%); different from VZV embryopathy or congenital varicella syndrome which occurs in first 20 weeks of pregnancy
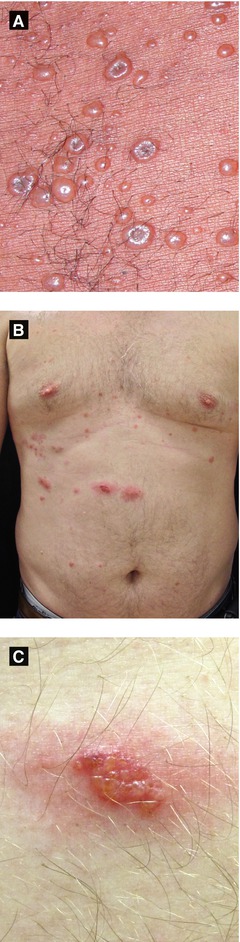
Figure 4.3:
A: Disseminated varicella in HIV patient(Courtesy of Dr. Sophie M. Worobec)B: Herpes zoster, trunkC: Herpes zoster, magnified
Epstein-Barr Virus (EBV, HHV4)
Infects B lymphocytes and establishes lifelong asymptomatic infection in these cells and mucosal epithelial cells
Causes infectious mononucleosis (IM), oral hairy leukoplakia (OHL), Gianotti-Crosti syndrome, Hodgkin’s lymphoma, endemic Burkitt’s lymphoma, post-transplant lymphoproliferative disorder (PTLD), nasopharyngeal carcinoma, and NK cell lymphoma
Of note, morbilliform eruption typically occurs in patient with mononucleosis if ampicillin or amoxicillin given
Cytomegalovirus (CMV, HHV5)
Asymptomatic/subclinical infection in healthy persons, but severe infections in infants infected before birth and immunosuppressed patients (especially with HIV or organ transplant); transmission via body fluids
Immunosuppressed patients: infection can lead to ocular (CMV necrotizing retinitis), CNS (meningoencephalitis), GI tract (inflammation with painful ulcerations), and lung abnormalities (pneumonitis)
Presents with wide variation: asymptomatic or mono-like symptoms; polymorphous eruption including vesicles, nodules, or verrucous plaques
Histology: cytomegalic endothelial and/or epithelial cells enlarged with intranuclear inclusions, eccentrically displaced nucleus with halo (‘owl’s eye’ inclusion bodies)
In AIDS can present with chronic perianal and lower extremity ulcerations, esophagitis, pneumonitis, chorioretinitis
Treatment of choice is ganciclovir
HHV6
Etiologic agent of exanthem subitum (roseola infantum or sixth disease)
Transmission via saliva with lifelong latency after primary infection
Complications infrequent in healthy patients: most common include febrile seizures
Treatment: no treatment required in healthy patients
HHV7
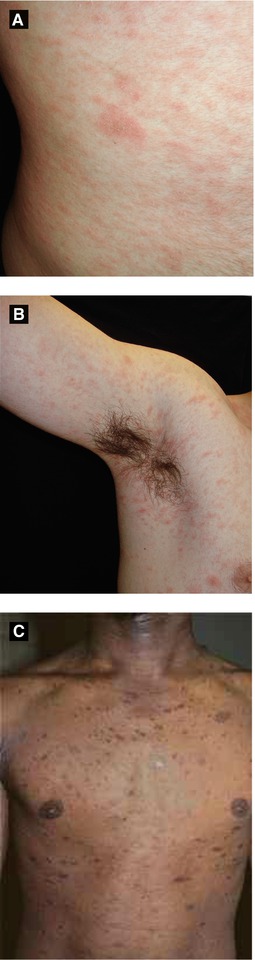
Figure 4.4:
A: Pityriasis rosea, herald patchB: Pityriasis roseaC: Pityriasis rosea(Courtesy of Dr. Paul Getz)
Etiologic agent of all types of Kaposi’s sarcoma (KS)
Four types of KS:
Classic: indolent, purple-red plaques on lower extremities in elderly men from Mediterranean descent, slow progression, rare involvement of GI tract and oral mucosa
AIDS-related: widely-distributed purpuric macules, patches and plaques on skin, oral and genital mucosa, GI tract and lung
Immunosuppression-associated: similar to AIDS-related KS with aggressive nature and dissemination
African endemic: aggressive, young patients in equatorial Africa, unrelated to HIV, subtypes include nodular, lymphadenopathic, florid and infiltrative
Other conditions associated with HHV8: Castleman’s disease (non-malignant lymphoproliferative disorder) and primary effusion lymphoma
Histology: spindle cells forming slit-like vascular channels with surrounding hemosiderin, promontory sign
Treatment: topical retinoid, surgery, radiotherapy, cryotherapy, systemic chemotherapy for extensive disease, HAART if AIDS-related
B. HUMAN PAPILLOMAVIRUS (HPV) (Table 4-1)
Non-enveloped dsDNA virus with more than 100 different HPV types; infects epithelia and mucosa
Genome encodes ‘E’ (early) and ‘L’ (late) proteins
‘E’ proteins (E1-E7) code for viral DNA replication; E6 and E7 oncogenes lead to keratinocyte immortalization; low levels expressed in basal layer
‘L’ proteins (L1 and L2) code for viral structural proteins (form outer shell: virion), expressed in superficial epithelium
Transmission mainly via direct skin contact, less likely via fomites; basal keratinocyte target of HPV (long-term reservoir of viral DNA)
Divided into nongenital and genital infections; also divided into benign or low risk (HPV 6/11) and high risk (HPV 16/18) types
Gardasil® vaccine: composed of L1 capsid protein with 4 types of recombinant HPV (type 6, 11, 16, 18)
Cervarix® vaccine: L1 protein for HPV 16 and 18
Clinical manifestations of HPV infection:
Common, plantar, and flat warts (Figure 4.5C)
Condyloma acuminata: lesions without significant scale in genital area
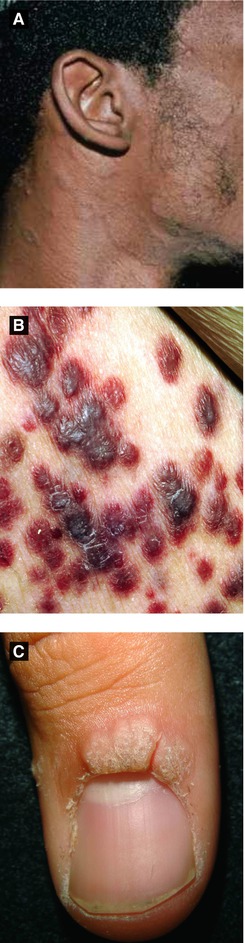
Figure 4.5:
A: Pityriasis rosea, face(Courtesy of Dr. Paul Getz)B: Kaposi’s sarcoma(Courtesy of National Cancer Institute)C: Verruca vulgaris
Table 4-1:
HPV Subtypes
Lesion | HPV types (frequent) | HPV types (less frequent) |
|---|---|---|
Common wart | 1, 2, 4 | 26, 27, 29, 41, 57, 60, 63, 65 |
Plantar wart | 1 | 2, 4, 63 |
Flat wart | 3, 10 | 28, 29 |
Butcher’s wart | 2, 7 | 1, 2, 3, 4, 10, 28 |
Epidermodysplasia verruciformis (EV) | 2, 3, 5, 8–10, 12, 14, 15, 17 | 19–25, 36–38, 46, 47, 49, 50 |
Focal epithelial hyperplasia (Heck’s) | 13, 32 | |
Verrucous carcinoma | 6, 11 | |
Condyloma acuminata | 6, 11 | 40, 42–44, 51, 54, 55, 61, 70, 72, 81 |
Bowenoid papulosis | 16, 18 | 26, 31, 33, 35, 39, 45, 51–53, 56, 58, 59, 62, 66, 68, 73 |
Digital SCC | 16 | 34, 35 |
SCC (in EV) | 5, 8 | 14, 17, 20, 47 |
Cervical cancer | 16, 18 | 31, 33, 35, 39, 45, 51, 52, 56, 58, 66, 68, 70 |
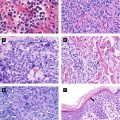
Bowenoid papulosis: red-brown papules or plaques involving genital and/or perineal area (clinically appear as genital warts, but histology consistent with Bowen disease) (Figure 4.6A)
Verrucous carcinoma: ‘semi-malignant’ (Figure 4.6B-D)
Florid oral papillomatosis: widespread verrucous carcinoma in oral cavity
Buschke-Lowenstein tumor: large cauliflower-like tumor of anorectum and external genital, focal malignant transformation may occur
Epithelioma cuniculatum of sole: slow-growing warty mass on sole
Focal epithelial hyperplasia (Heck’s disease): papules on buccal, gingival, labial mucosa resembling flat warts
Epidermodysplasia verruciformis: sporadic or AR inheritance, abnormal susceptibility of skin to HPV; red-brown macules with mild scale on face/trunk or flat-topped papules on hands resembling flat warts (malignant transformation in 50% patients)
Histology: papillomatosis, massive orthokeratosis, columns of parakeratosis, coarse keratohyalin granules of variable size, vacuolated cells (koilocytes), dilated and thrombosed capillaries
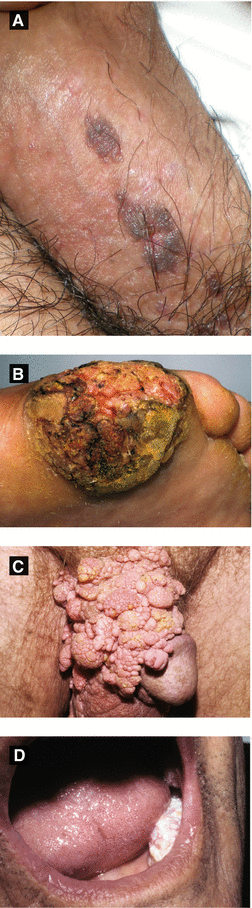
Figure 4.6:
A: Bowenoid papulosis*B: Epithelioma cuniculatum of sole*C: Buschke-Lowenstein tumor*D: Florid oral papillomatosis**Reprint from Baykal C, Yazganoğlu KD (eds). Clinical Atlas of Skin Tumors. Heidelberg, Germany. Springer; 2014.
C. POXVIRUS INFECTIONS
Table 4-2:
Select Poxvirus Infections
Disease | Virus | Clinical Findings | Treatment | Comments |
|---|---|---|---|---|
Molluscum Contagiosum | Molluscipox ↓ Molluscum contagiosum virus | Umbilicated pink, firm waxy papules seen mainly in children If adult with genital lesions, likely sexual transmission Larger lesions seen in patients with AIDS | Usually self-limited Treatment: cantharidin, cryosurgery, curettage, imiquimod | Henderson- Patterson molluscum bodies (intracytoplasmic inclusion bodies) |
Orf (Contagious Pustular Dermatosis) (Ecthyma Contagiosum) (Figure 4.7A) | Parapox ↓ Orf virus | One to few papules at contact site with infected goat/sheep, ± fever, lymphadenitis; 6 clinical stages (in order: maculopapular, targetoid, acute, regenerative, papillomatous, regressive) | Supportive treatment as self-limited | Mainly in shepherds, veterinarians, goat herders, and butchers |
Milker’s nodule (Pseudocowpox) (Paravaccinia) | Parapox ↓ Paravaccinia virus | Presents as solitary red-purple nodule on finger with slow growth or with multiple cherry-red nodules at inoculation site | Supportive treatment as self-limited | Recent contact with infected cows, calves, or viral fomites |
Vaccinia | Orthopox ↓ Vaccinia virus | Local reaction to site of vaccination (erythema or pruritic papule) Eczema vaccinatum (in atopic patients): diffuse infection in eczematous skin | Supportive; heals with pitted scarring | Live virus used for smallpox vaccine |
Smallpox (Figure 4.7B) | Orthopox ↓ Variola virus | Prodrome (backache, fever) after incubation period (12–14 days) Macules/papules initially on face, spreads to trunk and extremities → papules turn to vesicles/pustules with central umbilication | Respiratory and contact isolation, vaccination if early | All lesions same stage of development Transmission via respiratory droplets |
Cowpox | Orthopox ↓ Cowpox virus | Painful inflamed macule or papule at contact site with infected cow → vesicular, then pustular with tendency to ulcerate → deep-seated black eschar with erythema | Supportive as self-limited; heals with scarring | Eschar with surrounding edema/erythema similar to cutaneous anthrax |
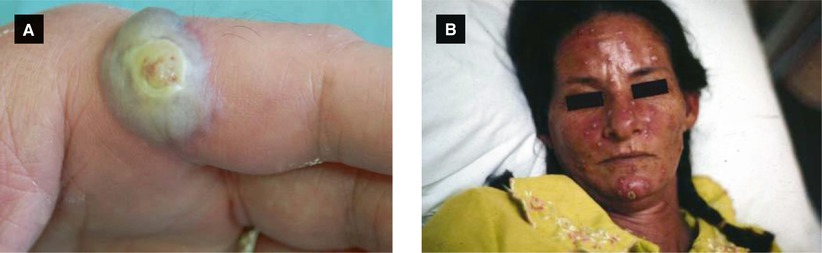
Figure 4.7:
A: Orf* B: Smallpox***Reprint from Lipsker D (ed). Clinical Examination and Differential Diagnosis of Skin Lesions. Paris, France; Springer; 2013.**Reprint from Morgan MB, Smoller BR, Somach SC (eds). Deadly Dermatologic Diseases. New York, NY: Springer; 2007.
D. MISCELLANEOUS (Tables 4-3 and 4-4)
Table 4-3:
Classification of Viruses
RNA | dsDNA | ssDNA |
|---|---|---|
Togavirus (rubella) | Herpesvirus (HSV, CMV, EBV, KSHV) | Parvovirus |
Flavivirus (HCV, dengue fever, yellow fever) | Hepadnavirus (HBV) | |
Orthomyxovirus (influenza) | Adenovirus | |
Rhabdovirus (rabies) | Papovavirus (papillomavirus, JC virus) | |
Picornavirus (rhinovirus, hepatovirus [HAV], enterovirus [poliovirus, enterovirus, coxsackievirus, echovirus]) | Poxvirus (molluscipox, orthopox, parapox) | |
Paramyxovirus (measles, mumps, RSV) | DNA virus mnemonic: HHAPPPy | |
Retrovirus (HIV, HTLV) |
Table 4-4:
Select Vaccinations
Live Attenuated Virus | Killed Virus | Purified Products |
|---|---|---|
Influenza (nasal spray, FluMist®) | Influenza (injection) | Pneumococcus (Pneumovax®) |
Yellow fever | Rabies | Tetanus |
Typhoid (oral) | Typhoid (injection) | Hepatitis B |
Polio (oral) | Polio (injection) | Diphtheria |
Rubella | Hepatitis A | HPV (Gardasil®) |
Mumps | Cholera | HPV (Cervarix®) |
Measles | Bubonic plaque | |
BCG (M. bovis) | ||
VZV (Zostavax®) |
Live Virus: ROMe Is MY Best Vacation: rubella, oral polio, mumps, influenza, measles, yellow fever, bcg, varicella
Killed/Purified Virus:
Rest in PPPeace Always:
rabies, influenza, polio (injection), pneumococcus, papillomavirus, A hepatitis (and B)
4.2 Bacterial Infections
A. GRAM-POSITIVE INFECTIONS
STAPHYLOCOCCAL INFECTIONS:
S. aureus: aerobic, gram-positive catalase-positive bacteria arranged in clusters
Best defense: intact skin
MRSA: ↑ resistance to methicillin caused by staphylococcal chromosome cassette mec (SCC mec), specifically mecA gene (encodes alternative penicillin-binding protein, PBP2a)
Select S. aureus toxins:
Toxic shock syndrome toxin-1 (TSST-1) | Superantigen, involved in toxic shock syndrome (TSS) |
Exfoliative toxin (ET-A, ET-B) | Protease activity, splits epidermal desmoglein 1, involved in staphylococcal scalded skin syndrome (SSSS) and bullous impetigo |
Panton-Valentine leukocidin (PVL) | In many community acquired MRSA strains, associated with ↑ virulence (leukocyte destruction, necrosis) |
Impetigo (Figure 4.8A-B)
Highly contagious infection seen primarily in children
Two types: bullous and nonbullous
Nonbullous: S. aureus most common cause, less common Gr. A strep (GAS)
Erythematous macule → pustule/vesicle → erosion with golden crust (+ culture from exudate under crust)
Bullous: S. aureus ONLY (usually phage II, type 71)
Flaccid, transparent bullae → rupture leaving shiny, dry erosion with no surrounding erythema, ± fever, diarrhea, weakness
Cleavage at granular layer due to ET (A/B) binding to desmoglein 1; S. aureus at site of lesion
Unlike SSSS
Treat with topical mupirocin, if extensive can use oral antibiotic (ie. cephalexin, dicloxacillin, etc)
Exfoliative disease mainly in neonates and young children; can occur in adults with renal insufficiency or if immunocompromised (mortality > 50%)
Presents with fever, conjunctivitis, initial tenderness of skin and erythema over body folds → generalized wrinkled appearance with subsequent exfoliation (‘sad man’ facies),
perioral crusting/fissuring, + Nikolsky sign
S. aureus phage II (types 3A, 3C, 55 or 71) at a distant site (extralesional): ET (A/B) → binds desmoglein 1 in granular layer causing superficial bulla
Culture of bullae → negative (infection at remote site)
Treatment: penicillinase-resistant penicillin (ie. dicloxacillin) and IV fluid support
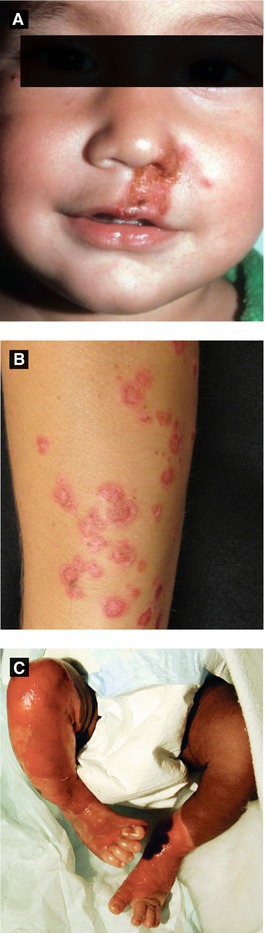
Figure 4.8:
A: Impetigo(Courtesy of Dr. Paul Getz)B: Bullous impetigo, armC: SSSS(Reprint from Allen HB. Dermatology Terminology. New York, NY: Springer; 2010.)
Multisystem illness due to S. aureus, initially in women with use of superabsorbent tampons, but now more commonly seen in infections with wounds, catheters, deep abscesses, or nasal packing
Superantigen-mediated TSST-1 results in polyclonal T cell activation → cytokine storm (TNF, IL-1, etc)
Presentation:
Four criteria: fever, hypotension, macular exanthem, and involvement of three or more organ systems
Exanthem: diffuse scarlatiniform exanthem on trunk spreading outward, palmoplantar edema and erythema (with desquamatiion 1-3 weeks later), hyperemia of conjunctiva
Treatment: remove any nidus of infection, parenteral β-lactamase resistant antibiotic, and fluid support
Bacterial Folliculitis
Superficial infection of hair follicle usually due to S. aureus
Presents with pustules in follicular distribution associated with hairs
Treatment: antibacterial wash (chlorhexidine or triclosan), antibacterial ointments (mupirocin), and if widespread can use oral antibiotic
Furuncle, Carbuncle, Abscess (Figure 4.9B-C)
Typically due to S. aureus
Depth of infection determines presentation
Furuncle: deep-seated tender nodule of hair follicle
Carbuncle: coalescing of adjacent furuncles with multiple draining sinuses (typically involves nape of neck or back of thighs)
Abscess: inflamed walled off collection of pus (Figure 4.10A)
Treatment
Simple furuncle (no fluctuance): warm compresses
Fluctuant furuncle or abscess: incision and drainage
Oral antibiotics if:
Located near midface (concern for cavernous sinus thrombosis) or external auditory canal
Recurrent or recalcitrant to local area alone
Very large or with surrounding cellulitis
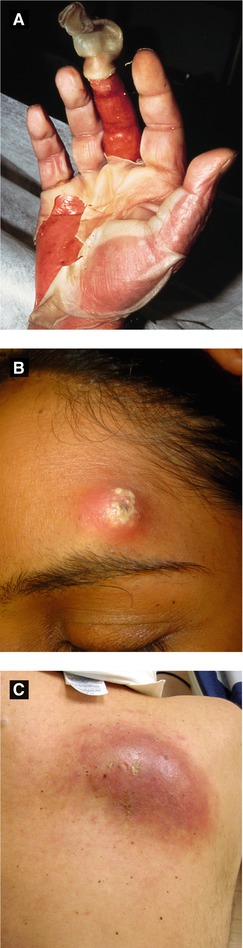
Figure 4.9:
A: Toxic shock syndrome(Reprint from Morgan MB, Smoller BR, Somach SC. Deadly Dermatologic Diseases. New York, NY: Springer; 2007.)B: FuruncleC: Carbuncle
STREPTOCOCCAL INFECTIONS
Gram-positive bacteria arranged in chains or pairs
Not part of normal cutaneous flora (but resident of aerodigestive tract and vagina)
Classification via two methods:
Ability to induce hemolysis (α, β, γ) and/or
Lancefield groups (A-D, G) based on characteristic polysaccharide cell wall
Of note, group A β-hemolytic streptococci (S. pyogenes, GAS) most pathogenic
The following antibodies become positive after infection with GAS: anti-streptolysin O (ASO), anti-hyaluronidase, and anti-DNase-B
Certain strains with erythrogenic toxins: S. pyogenes exotoxins (SPE-A, SPE-B, SPE-C)
Cellulitis (Figure 4.10B)
Infection of the deep dermis and subcutaneous tissue, mostly due to GAS (S. aureus less common)
In immunocompetent patients, usually first step is a break in the skin barrier
Presents as an ill-defined area with erythema, swelling and tenderness, ± fever, chills
Treatment: oral antibiotic with good Gram-positive coverage
Superficial type of cellulitis with significant dermal lymphatic involvement; typically due to GAS
Presents as a well-defined, bright red indurated plaque with sharp, raised borders commonly on the face or legs, ± constitutional symptoms
Treatment of choice: PCN (if PCN-allergic can use macrolide)
Blistering Distal Dactylitis
Unique GAS bullous eruption in children
Tense stable blisters on tender erythematous base over volar tips of toes or fingers
Treatment: dicloxacillin or first-generation cephalosporin
Necrotizing Fasciitis
Rapidly progressive necrosis of subcutaneous tissue and fascia due to GAS, but typically mixed infection with 30% mortality rate
Risk factors include advanced age, diabetes, peripheral vascular disease and/or history of alcohol abuse
Presents as tender, erythematous tense plaques recalcitrant to antibiotics and progresses at an alarming rate → necrosis of fascia and fat renders watery foul-smelling fluid
Treatment: extensive surgical debridement
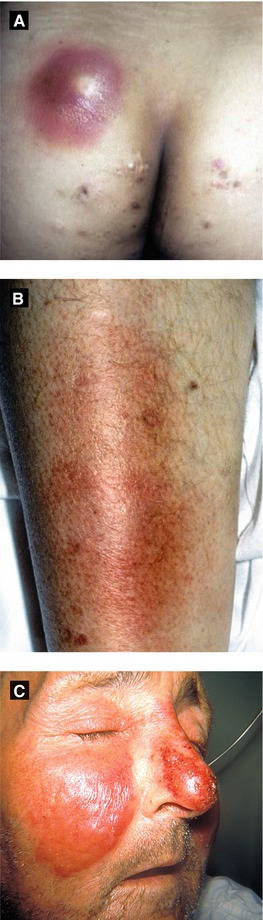
Figure 4.10:
A: Abscess(Courtesy of Dr. Paul Getz)B: Cellulitis(Courtesy of Dr. Paul Getz)C: Erysipelas(Courtesy of CDC: Dr. Thomas Sellers, Emory University)
Perianal Streptococcal Disease (Figure 4.11A)
Perianal GAS infection typically in preschool children
Presents with circular band of erythema around anus, ± painful defecation, blood-streaked stools, anal leakage
Obtain both throat and perianal culture; treat with PCN or erythromycin × 10-14 days
Do not confuse with ecthyma gangrenosum
Ecthyma (Figure 4.11B)
Deeper form of nonbullous impetigo with ulceration due to GAS but quickly contaminated by S. aureus
Presents as ‘punched out’ shallow ulcer with thick, yellow-gray crust commonly in lower legs of children
If diagnosis uncertain → punch biopsy with deep-tissue Gram stain and culture
Treatment: dicloxacillin or first generation cephalosporin
Scarlet Fever (Figure 4.11C)
Diffuse exanthem from GAS pharyngitis with erythrogenic toxin (SPE-A, B, C); mainly in children
Presents with sore throat, headache, fever → tiny pink papules on erythematous background (sandpaper-like), linear petechiael streaks along body folds (Pastia’s lines), circumoral pallor, palatal petechiae, ‘strawberry tongue’ initially white then red
Treatment: PCN or erythromycin × 10-14 days
Streptococcal Toxic Shock Syndrome (STSS)
Rapidly progressive multi-organ illness, high mortality (30-60%), caused by GAS
Superantigen mediated: SPE-A → stimulates T cells with massive cytokine release → subsequent shock
Presents typically with sudden onset pain in an infected soft tissue, flu-like symptoms, CNS symptoms (confusion, coma) → multi-organ failure
Generalized exanthem less common in STSS (vs. TSS), and STSS more likely in an otherwise healthy adult
Treatment: intensive supportive therapy, IV penicillinase-resistant PCN or oral clindamycin (latter may more rapidly shut down toxin production)
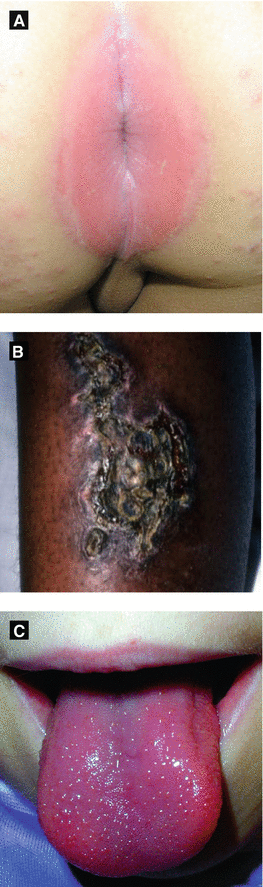
Figure 4.11:
A: Perianal strep(Reprint from Al-Jasser M, Al-Khenaizan S. Cutaneous mimickers of child abuse. Eur J of Ped. 2008; 167(11): 1221-30.)B: Ecthyma(Courtesy of Dr. Paul Getz)C: Strawberry tongue(Reprint from Allen HB. Dermatology Terminology. New York, NY: Springer; 2010.)
CORYNEBACTERIAL INFECTIONS
Corynebacterium: gram-positive rod-shaped bacteria
Erythrasma (Figure 4.12A-B)
Superficial infection in occluded intertriginous areas due to C. minutissimum
Presents as well-demarcated red-brown macules/patches with fine scale and wrinkling in intertriginous areas; interdigital maceration and scaling between toes
Most common bacterial infection of the foot
Wood’s lamp: bright coral-red fluorescence due to porphyrin production (coproporphyrin III)
Treatment: topical antibiotic or antifungal (clindamycin, erythromycin, imidazole) or oral erythromycin × 5 days
Trichomycosis Axillaris
Superficial bacterial colonization with C. tenuis of hair shafts in axilla; ↑ risk with hyperhidrosis/poor hygiene
Presents with white-yellow, red or black adherent nodules attached to hair shafts (‘frosted’ appearance of hairs) with characteristic rancid acidic odor, common in axilla and rarely affects pubic area
Treatment: shave axillary hair, topical benzoyl peroxide or topical clindamycin
Pitted Keratolysis (Figure 4.12C-D)
Non-inflammatory infection due to Corynebacteria spp. or Kytococcus sedentarius (previously called Micrococcus)
Bacteria produce keratin-degrading proteases
Presents with asymptomatic shallow crater-like depressions over weight-bearing areas of feet, accompanying hyperhidrosis and malodor
Treatment: topical clindamycin, erythromycin or benzoyl peroxide
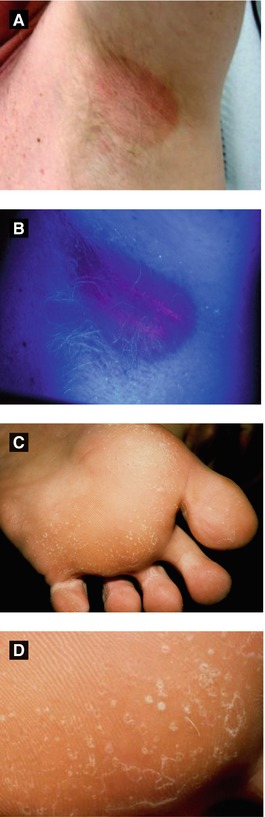
Figure 4.12:
A: ErythrasmaB: Erythrasma (Wood’s light)C: Pitted keratolysis(Courtesy of Dr. Paul Getz)D: Pitted keratolysis(Courtesy of Dr. Paul Getz)
Cutaneous Diphtheria (Figure 4.13A)
Localized infection of C. diphtheriae, endemic in several tropical countries, skin involvement via inoculation to an otherwise insignificant wound
Presents as sharply bordered, punched out ulcer with yellow leathery pseudomembrane (primary disease) or pre-existing wound becomes infected (secondary disease)
If toxin produced, risk of cardiac or neurologic disease
Treatment: diphtheria antitoxin from horse serum (before toxin binds cells) crucial, PCN or erythromycin × 10-14 days
Other Gram-Positive Infections
Acute disease in humans and animals caused by Bacillus anthracis, a Gram-positive spore-forming rod
Clinical forms: cutaneous, pulmonary, and GI
Cutaneous form: ‘malignant pustule’ at inoculation site which spreads and becomes hemorrhagic → central eschar with surrounding nonpitting edema → eschar sloughs leaving shallow ulceration
Virulence factors: capsule and 2 exotoxins: edema toxin (increases cAMP levels) and lethal toxin (increases TNFα and IL1β promoting shock/death)
Bioterrorism-associated treatment: ciprofloxacin or doxycycline (conventional treatment: PCN)
Infection caused by Erysipelothrix rhusiopathiae through direct contact with infected meat, seen mainly in meat-handlers, fisherman or veterinarians
Presents with painful red to purple patches over hands (finger webs often involved, sparing terminal phalanges) with sharply-marginated spreading edge, possible central clearing and/or hemorrhagic vesicles
Systemic form with fever, arthralgias, widespreaed cutaneous lesions, possible sepsis and fatal endocarditis
Treatment: penicillin (if PCN allergy → erythromycin)
Gas Gangrene (Clostridial Myonecrosis)
Most severe form of infectious gangrene developing in deep lacerated wounds of muscle tissue caused by Clostridium spp. and subacute variety due to other bacteria
Presents with severe localized pain with sudden onset and accompanying fever/chills; physical exam shows crepitation on palpation and characteristic mousy odor
Treatment is wide surgical debridement and extensive antibiotic therapy
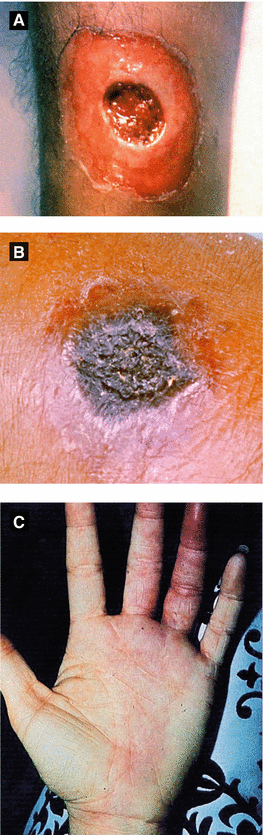
Figure 4.13:
A: Cutaneous diphtheria(Courtesy of Public Health Image Library: CDC)B: Cutaneous anthrax(Courtesy of James Steele, CDC)C: Erysipeloid(Reprint from Mandell G, ed. Atlas of Infectious Diseases. Philadelphia, PA: Current Medicine LLC; 2002.)
Due to Actinomyces israelii, an anaerobic filamentous Gram-positive bacteria; part of normal oral flora
Risk factors: poor dental hygiene, dental procedures, traumatic injuries
Presents as a firm nodule or bluish swelling at angle of jaw → direct spread into adjacent tissues → formation of fistulas discharging purulent material with granules (yellow sulfur-like appearance consisting of masses of bacteria, both gram-negative and gram-positive)
Treatment: intravenous PCN initially, then switch to oral PCN × 6-12 months
Suppurative infection caused by bacteria (actinomycetoma) or fungus (eumycetoma)
Bacterial infection due to Nocardia brasiliensis, Nocardia asteroides , Actinomadura madurae, Actinomadura pelletieri , Streptomyces somaliensis
Presents as painless nodules at site of trauma (typically on foot) → increases in size with purulence, tumefaction, draining sinuses and exudate containing grains
N. brasiliensis → primary cutaneous nocardiosis (lymphocutaneous infection, mycetoma or superficial infection [ulceration, abscess, cellulitis])
N. asteroides → disseminated nocardiosis (10% disseminated to skin in patients with systemic nocardial infection)
Treatment: surgical debridement/azole antifungal (eumycotic) or bactrim/streptomycin (actinomycotic)
Grain Color | Organism |
|---|---|
White | Nocardia brasiliensis, Nocardia asteroides |
Pink or cream | Actinomadura madurae |
Yellow to brown | Streptomyces somaliensis |
Red | Actinomadura pelletieri |
B. GRAM-NEGATIVE INFECTIONS
PSEUDOMONAL INFECTIONS
Pseudomonas aeruginosa: gram-negative bacteria, grows well in aqueous environment, has ability to produce variety of pigments:°Greenish-blue pyocyanin° Yellow-green fluorescein° Brown-black pyomelanin
Green Nail Syndrome (Figure 4.14D)
Subungual pseudomonal infection causing green discoloration of nail and onycholysis
Treatment: trim nail, acetic acid soaks, topical ciprofloxacin or thymol solution
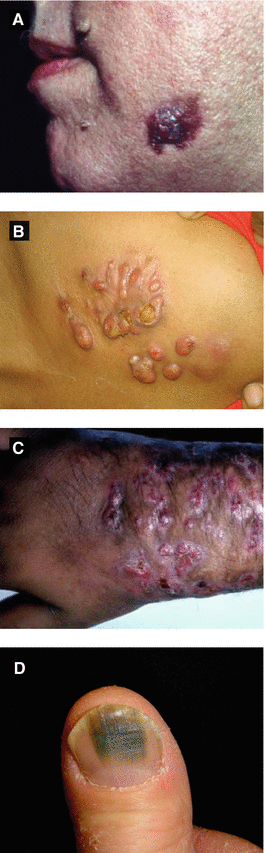
Figure 4.14:
A: Actinomycosis(Courtesy of Dr. Paul Getz)B: Actinomycosis, chest(Courtesy of Dr. Vandana Mehta, India)C: Actinomycetoma, arm(Courtesy of Dr. Paul Getz)D: Green nail syndrome
Ecthyma Gangrenosum (Figure 4.15A)
Cutaneous manifestation of severe, invasive infection by P. aeruginosa typically in immunosuppressed patients
Presents initially as erythematous macules → opalescent, tense vesicles or pustules → hemorrhagic and violaceous vesicles → rupture and form ulcers with necrotic centers
Treatment: intravenous aminoglycoside with anti-pseudomonal penicillin
Pseudomonas Hot Foot Syndrome
Painful plantar purple-red nodules after exposure to pool water contaminated with P. aeruginosa
Self-limited
Pseudomonal Folliculitis (Hot Tub Folliculitis)
Folliculitis due to nonpathogenic strain of P. aeruginosa
Presents with erythematous follicular papules and pustules at sites of exposure to water (via whirlpool, hot tub, rarely swimming pool) with sparing of face and neck
Self-limited in immunocompetent person
Pseudomonal Pyoderma
Superficial infection of skin with P. aeruginosa with ‘mousy’ odor
Presents typically on feet with macerated ‘moth-eaten’ appearance, green-blue purulence and eroded borders
Blastomycosis-like pyoderma presents as verrucous plaques with elevated borders and pustules as a chronic vegetating infection
Other Gram-Negative Infections
Acute Meningococcemia (Figure 4.15B-C)
Acute and potentially life-threatening infection of the blood vessels caused by Neisseria meningitidis, an encapsulated gram-negative diplococcus
Bacterial carriage via nasopharynx
Presents initially with erythematous macules/papules → evolve to stellate purpuric patches/plaques with ischemic necrosis and/or hemorrhage, accompanied by high fever and toxic appearance
Recurrent infections in patients with defects in late components of complement (C5-C9)
Treatment: high dose IV PCN (if resistant, use 3rd generation cephalosporin)
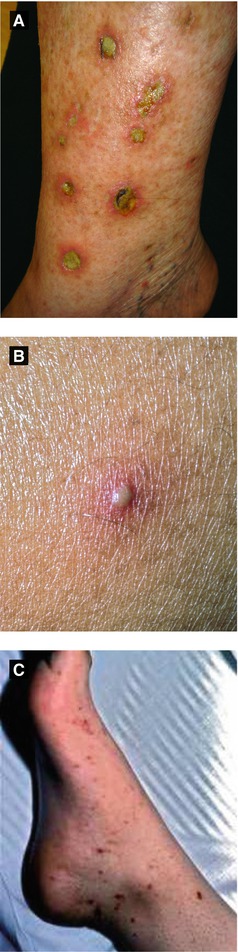
Figure 4.15:
A: Ecthyma gangrenosumB: Meningococcemia(Courtesy of Dr. Paul Getz)C: Meningococcemia(Courtesy of Dr. Paul Getz)
Table 4-5:
Select Gram-Negative Infections
Disease | Etiology/Vector | Clinical Findings | Treatment |
|---|---|---|---|
Glanders | Burkholderia mallei Contact with infected horses | Ulcerated nodule at inoculation site with regional lymphadenopathy, ± ‘farcy buds’ (nodules along lymph nodes) | Sulfonamide |
Brucellosis (Undulant fever) (Malta fever) | Brucella spp. Direct contact with infected animal or ingestion of dairy (unpasteurized)/infected meat | Cyclic fevers, arthralgias, hepatospleno-megaly; rare skin involvement (violaceous papulonodular eruption) ↑ Risk: butchers, farmers, veterinarians | Doxycycline combined with rifampin |
Tularemia (Rabbit fever) (Deer fly fever) | Francisella tularensis Direct contact with wild animals like rabbits (rabbit-borne), ticks (tick-borne) or deer flies | Ulceroglandular: tender chancre-like papule or nodule with lymphadenopathy, lymph nodes may become fluctuant with suppuration ↑ Risk in hunters | Streptomycin |
Vibrio infection | Vibrio vulnificus Ingestion of raw seafood or open wound exposed to seawater | Fever, chills, abdominal pain, red to violaceous macules → painful hemorrhagic bullae with cellulitis ↑ Risk: diabetes, liver disease, immunosuppression | Oral TCN |
Plague | Yersinia pestis Transmitted via flea bite from infected animals | Myalgias, malaise, fever → small papule/pustule at site of flea bite with swollen, painful fluctuant lymph nodes (‘buboes’) | Streptomycin (IM) |
Malakoplakia | E. coli (± Pseudomonas aeruginosa, Proteus, Klebsiella) | Commonly affects urinary tract, rare skin involvement with weeping perianal plaque or polypoid mass Histo: Michaelis-Gutmann bodies | Cipro (long-term) or surgical removal |
Rhinoscleroma | Klebsiella rhinoscleromatis Transmission via inhalation of droplets or contaminated material | Infectious granulomas in nasal mucosa and respiratory tract, epistaxis, Hebra nose (destruction of nasal cartilage) Histo: Mikulicz cell, Russell bodies | Cipro |
Rat-Bite Fever (Haverhill fever) | Streptobacillus moniliformis Direct contact from rodents or contaminated food | Fever, arthritis, ± ulceration at site of bite and generalized morbilliform eruption with acral distribution | Penicillin |
Cat Bite | Pasteurella multocida | Erythema, pain, tenderness with gray serous drainage from puncture wound | Augmentin, irrigate site, ± tetanus prophylaxis |
Dog Bite | Capnocytophaga canimorsus Pasteurella canis Pasturella multocida | ||
Human Bite | Eikenella corrodens |
Bartonella Infections
Gram-negative, facultative intracellular bacteria
Can infect healthy individuals but considered especially important as an opportunistic pathogen
Transmitted via insect vectors (ticks, fleas, sand flies, and mosquitoes)
Adheres to and invades erythrocytes (B. bacilliformis)
All three types can produce an endothelial cell-stimulating factor → proliferation of both endothelial cells and blood vessels
Table 4-6:
Select Bartonella Infections
Disease | Etiology/Vector | Clinical Findings | Treatment |
|---|---|---|---|
Oroya Fever (Carrion’s disease) (Verruga peruana) (Figure 4.16A) (Peruvian wart) | Bartonella bacilliformis Vector: sandfly Lutzomyia verrucarum | Biphasic disease: – Acute stage (Oroya fever): fever + hemolytic anemia – Chronic stage (verruga peruana): erythematous papules/nodules, resolves spontaneously but may persist for years | Acute stage: chloramphenicol (covers salmonella coinfection) Chronic stage: TCN or PCN |
Cat-Scratch Disease | Bartonella henselae Vector: cat flea Ctenocephalides felis Transmission via cat bite or scratch (flea feces inoculated into scratch site) | Unilateral tender lymphadenitis 2-4 weeks after cat scratch, typically in axilla > epitrochlear node (can last between 2–5 months) Parinaud oculoglandular syndrome: unilateral conjunctivitis and regional lymphadenitis Rare atypical manifestations: encephalitis, pneumonia, erythema nodosum, arthralgia, thrombocytopenic purpura | Spontaneous resolution typical If patient immunosuppressed treat with doxycycline or erythromycin |
Bacillary Angiomatosis | Bartonella henselae Bartonella quintana Vector: lice, ticks, fleas | Erythematous tender papules and nodules resembling pyogenic granulomas, seen mainly in HIV patients | Doxycycline or erythromycin |
Trench Fever (Shin bone fever) | Bartonella quintana Vector: body louse Pediculus humanus var. corporis Also causes epidemic typhus | Fever (relapsing), chills, tenderness of shins, back pain, and transient macular eruption ↑ Risk: crowding and poor hygiene | Doxycycline or erythromycin |
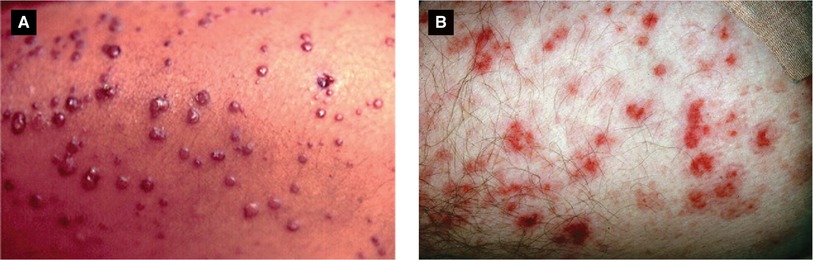
Figure 4.16:
A: Verruga peruana(Reprint from Silverberg NB, Durán-McKinster C, Tay Y-K (eds). Pediatric Skin of Color. New York, NY. Springer; 2015.)B: Rocky Mountain spotted fever(Reprint from Morgan MB, Smoller BR, Somach SC (eds). Deadly Dermatologic Diseases. New York, NY: Springer; 2007.)
Rickettsiae
Gram-negative, motile, pleomorphic bacteria; obligate intracellular parasite (usually infecting endothelial cells, causing vasculitis)
Bacteria carried as parasites by many ticks, fleas, and lice
Includes R. rickettsii , R. akari , R. conori , R. prowazekii , R. typhi , R. tsutsugamushi (latter reclassified into genus Orientia)
Few bacteria which are morphologically similar to Rickettsiae: Coxiella burnetii and Ehrlichia
Table 4-7:
Select Rickettsial Infections
Disease | Organism/Vector | Clinical Findings | Treatment |
|---|---|---|---|
Rocky Mountain Spotted Fever (RMSF) (Figure 4.16B) | R. rickettsii Vector: tick Dermacentor andersoni (wood tick in Western US) Dermacentor variabilis (dog tick in Eastern US) | Fever, headache, myalgias → purpuric or hemorrhagic macules and papules on wrists/ankles initially → spreads to trunk, hands, feet (‘spotless’ in 10-20% cases) Mortality 15-25% if untreated | Doxycycline preferred treatment (in pregnant patients may use chloramphenicol) |
Mediterranean Spotted Fever(Boutonneuse fever) | R. conorii Vector: brown dog tick Rhipicephalus sanguineus | Two forms: – Tache noir: indurated papule at site of tick bite → necrotic ulcer – Exanthem: erythematous papules mainly over lower limbs | Doxycycline or chloramphenicol |
Rickettsialpox | R. akari Vector: mouse mite Liponyssoides sanguineus (formerly Allodermanyssus) | Initial papule or vesicle at site of bite → Eschar, regional lymphadenopathy → Sudden-onset fever, chills, headache, diffuse vesicular rash (self-limited) | Doxycycline or chloramphenicol |
Epidemic Typhus (Louse-borne) | R. prowazekii Vector: body louse Pediculosis humanus var. corporis Reservoir: flying squirrel | Fever, chills, headache → pale red macules on trunk → evolve to petechiael papules, spread to rest of body (spare face, palms, and soles) Vascular inflammation of skin, CNS, heart, kidneys and muscle | Doxycycline or chloramphenicol |
Endemic Typhus (Flea-borne) (Murine typhus) | R. typhi Vector: rat flea Xenopsylla cheopis | Fever, headache, myalgias with transient truncal maculopapular exanthem | Doxycycline or choramphenicol |
Scrub Typhus (Mite-borne typhus) | R. tsutsugamushi (now Orientia) Vector: chigger mites Trombiculid (larval stage) | Headache, chills, malaise and eschar at site of inoculation with lymphadenopathy → maculopapular rash on trunk, ± pulmonary and cardiac problems | Doxycycline or choramphenicol |
Ehrlichiosis (Monocytic (M) Ehrlichiosis) (Granulocytic (G) Ehrlichiosis) | E. chaffeensis (M) E. phagocytophilia (G) Vector: tick Ambyloma americanum (M); Ixodes scapularis, Ixodes pacificus (G) | Highly variable exanthem | Tetracycline or doxycycline |
Q Fever | Coxiella burnetii | No skin findings; limited febrile illness, severe headache, ± pneumonia, hepatitis, endocarditis | Doxycycline |
Spirochetes
Gram-negative bacteria with spiral-shaped cells, move via twisting motion (due to axial filaments in the flagella)
Include Treponema spp. , Borrelia spp. , and Leptospira spp.
Table 4-8:
Select Spirochete Infections
Disease | Organism/Vector | Clinical Findings | Treatment |
|---|---|---|---|
Lyme disease | B. burgdorferi Vector: tick Eastern US, Great Lakes Ixodes dammini (also known as I. scapularis) Western US Ixodes pacificus Europe Ixodes ricinus (reservoir: white-footed mouse) Principal source (reservoir) of bacteria is white-footed mouse, which tick (larva) feeds on and becomes infected → transmission to humans via infected tick saliva (of note, white-tailed deer is not reservoir for Lyme disease but principal host for adult ticks and are often infected) | Early localized disease: flu-like symptoms + erythema migrans: expanding erythematous patch at site of tick bite with central clearing, occurs ~1-2 weeks after tick bite, average diameter 5cm, disappears typically within 4 weeks without treatment Early disseminated disease: oval-shaped widespread patches (satellite erythema migrans lesions) due to spirochetemia, neural involvement (facial nerve common), migratory joint pain, carditis Chronic disease: persistent neurologic and rheumatologic symptoms, acrodermatitis chronic atrophicans: loss of subcutaneous fat with thin, atrophic skin | Diagnosis: PCR, tissue culture, serologic evidence Treatment: Adults, children (>8 y/o): Doxycycline × 14-21 days Pregnant women, children (<8 y/o): Amoxicillin × 14-21 days |
Borrelial lymphocytoma (Lymphocytoma cutis) | B. afzelli B. garinii (neither present in North America – only Europe) | Firm bluish-red tumor or plaque appears most commonly on ear lobes of children or nipple/areola in adults, less commonly involves genitalia, trunk or extremities | Doxycycline |
Relapsing fever (Louse-borne) | B. recurrentis Vector: body louse Pediculus humanus var. corporis | Paroxysmal fevers, myalgias, no specific cutaneous findings | Doxycycline |
Relapsing fever
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|