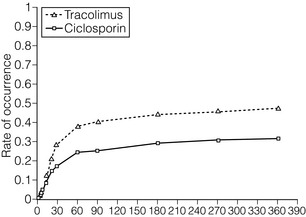
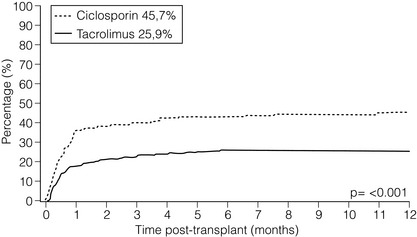
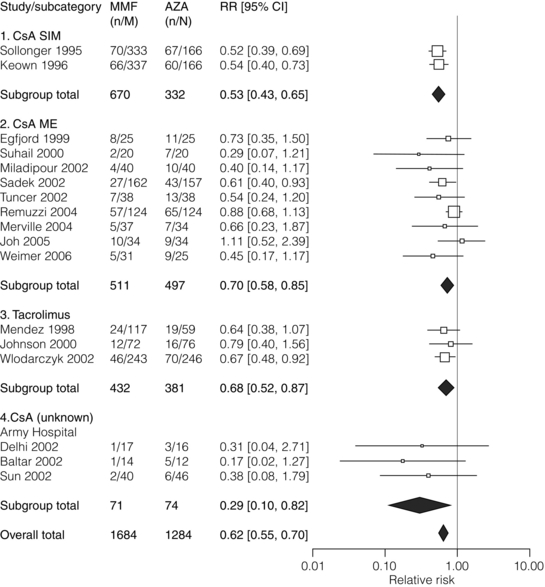
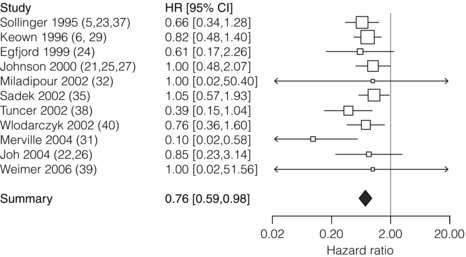
5 Unless a transplant is performed between identical twins, recipients of kidney transplants generally require lifelong immunosuppression to maintain a state of low immunoresponsiveness to the allograft. In the 1980s the calcineurin inhibitor1 ciclosporin was introduced; additional new small molecules and biologicals were successfully introduced in the clinic during the 1990s. Consequently, rejection rates, which were in the 40% range, fell to 10–15%.2 After the induction phase, transplant patients transition to the maintenance phase of immunosuppression.3 Effective maintenance immunosuppression is the key to preventing rejection throughout the life of the graft. Modern maintenance therapies include a calcineurin inhibitor (CNI) backbone along with antiproliferative agents (mycophenolate mofetil (MMF)/enteric-coated mycophenolate acid, azathioprine, or mammalian target of rapamycin (mTOR) inhibitors such as sirolimus and everolimus) and corticosteroids. The CNIs ciclosporin and tacrolimus are the backbone maintenance agents of current standard immunosuppressive regimens. Through calcineurin inhibition, both agents block downstream signalling, and thus activation, of T lymphocytes. Prior to the mid-1980s, options for immunosuppression following kidney transplantation included whole-body irradiation, polyclonal antilymphocyte antibodies, azathioprine and corticosteroids.4 With the introduction of ciclosporin, 1-year allograft survival rates improved from 50–60% to 70–80%.5 When used in combination with an antimetabolite and corticosteroids (triple-drug therapy), 1-year allograft survival rates of 80–90% are achieved. In 1984, the sentinel trial was published that supported the safety and efficacy of ciclosporin in renal transplant recipients.5 In this study, patients who received ciclosporin alone or azathioprine plus corticosteroids were compared. The 4-year allograft survival was 70% in the ciclosporin group and 62% in the azathioprine/steroid group. Over the subsequent 10 years, allograft survival was prolonged further by the use of triple drug therapy with ciclosporin, azathioprine and corticosteroids. Tacrolimus was FDA approved for liver transplant in 1994, and soon after was also being utilised in renal transplant recipients. Formerly known as FK-506, tacrolimus is a macrolide antibiotic isolated from the fungus Streptomyces tsukubaensis. It binds to FK-binding protein in the cytoplasm, which, similar to the ciclosporin/cyclophilin complex, blocks the activity of calcineurin, thus preventing proinflammatory cytokine transcription and T-lymphocyte activation.6 Both oral and intravenous forms of tacrolimus are available. Similar to the microemulsion forms of ciclosporin, the absorption of tacrolimus is not dependent on bile salts. Tacrolimus is a more potent immunosuppressant than ciclosporin; target trough levels range between 4 and 10 ng/mL. There have been numerous studies comparing the two CNIs. The first landmark trial was done in 1997 by the FK-506 Kidney Transplant Study Group.7 This study demonstrated a significant reduction in the incidence of biopsy-proven acute rejection (BPAR) in those recipients treated with tacrolimus-based immunosuppression (30.7% vs. 46.4%; Fig. 5.1). In the same year, the European Tacrolimus Multicentre Renal Study Group published similar results, with rates of BPAR in tacrolimus- and ciclosporin-treated groups of 25.9% and 45.7%, respectively (Fig. 5.2).8 In both of these trials, the 1-year allograft and patient survival rates were similar. Furthermore, these trials were both done using the non-microemulsion form of ciclosporin. In 2005, a large meta-analysis based on 30 trials was performed.9 In this analysis, tacrolimus was associated with improved allograft survival at 6 months and a decreased incidence of acute rejection in the first year. However, there are no direct convincing data showing that tacrolimus is associated with better outcomes than ciclosporin except possibly for slightly better renal function.10 Also, note that an advantage of tacrolimus is that managing drug levels is easier with tacrolimus as trough levels correlate better with the area under the curve (AUC) as compared to ciclosporin. Figure 5.1 There was a significant reduction in the incidence of biopsy-confirmed acute rejection in the tacrolimus-treated patients (30.7%) compared with the ciclosporin-treated patients (46.4%, P = 0.001). Reproduced from Pirsch JD, Miller J, Deierhoi MH et al. A comparison of tacrolimus (FK506) and cyclosporine for immunosuppression after cadaveric renal transplantation. Transplantation 1997; 63(7): 977–83. With permission from Wolters Kluwer Health. Figure 5.2 The 12-month Kaplan–Meier estimates of biopsy-proven acute rejection were 25.9% and 45.7% for the tacrolimus and ciclosporin treatment groups, respectively (P < 0.001; absolute difference: 19.8%, 95% confidence interval (CI): 10.0–29.6%). Reproduced from Mayer DA, Dmitrewski J, Squifflet JP et al. Multicenter randomized trial comparing tacrolimus (FK506) and cyclosporine in the prevention of renal allograft rejection: a report of the European Tacrolimus Multicenter Renal Study Group. Transplantation 1997; 64(3):436–43. With permission from Wolters Kluwer Health. The first antimetabolite widely used in kidney transplantation was azathioprine. Prior to the accepted use of ciclosporin, azathioprine along with corticosteroids was the maintenance agent of choice for nearly two decades. Once absorbed, azathioprine is converted to 6-mercaptopurine, which is the active form of the drug. The drug is available in both oral and intravenous forms, with the standard maintenance dosing of 1–3 mg/kg/day. Side-effects include malaise, fevers, nausea, anorexia, hepatotoxicity and leucopenia. Caution must be used in patients also on allopurinol, since the inhibition of xanthine oxidase will allow accumulation of the azathioprine active metabolites.11 MMF and mycophenolate sodium are newer antimetabolite agents used routinely in renal transplant recipients. Both agents are prodrugs and are rapidly metabolised to the active form, mycophenolic acid (MPA). MPA inhibits the enzyme inosine monophosphate dehydrogenase, which plays a key role in purine biosynthesis. MMF is available in both oral and intravenous forms. The typical starting dose for an adult patient is 1000 mg twice daily. Mycophenolate sodium is an enteric-coated formulation and is available only as an oral agent. MMF is teratogenic and is contraindicated in pregnancy.12 There remains controversy over the issue of which antimetabolite should be used as first line therapy in renal transplantation. Early studies suggested that MMF was superior to azathioprine since there were fewer acute rejection episodes.13,14 Arguably, these studies were done before the introduction of ciclosporin microemulsion formulations. In 2004, the Mycophenolate Mofetil versus Azathioprine for Prevention of Acute Rejection in Renal Transplantation (MYSS) trial was published,15 in which a ciclosporin microemulsion was used, and demonstrated no difference in the incidence of clinical rejection or rates of allograft loss. However, the cost of MMF was over 10-fold more than azathioprine. Conversely, a recent meta-analysis concluded that MMF, used in combination with any CNI, is superior to azathioprine, resulting in a lower incidence of acute rejection episodes (Fig. 5.3) and prolonged allograft survival (Fig. 5.4).16 In general, it is our standard practice to use MMF, in conjunction with tacrolimus. If patients develop gastrointestinal side-effects, we switch to mycophenolate sodium, which is occasionally better tolerated. Also, women who desire to get pregnant should be switched to azathioprine. Figure 5.3 The use of MMF significantly reduced the risk of acute rejection compared with azathioprine overall (relative risk 0.62; CI 0.55–0.70; P = 0.0001) with low heterogeneity (I2 = 6.8%). Reproduced from Knight SR, Russell NK, Barcena L et al. Mycophenolate mofetil decreases acute rejection and may improve graft survival in renal transplant recipients when compared with azathioprine: a systematic review. Transplantation 2009; 87(6):785–94. With permission from Wolters Kluwer Health. Figure 5.4 The hazard of graft loss was significantly reduced in the MMF group (hazard ratio 0.76, CI 0.59–0.98, P = 0.037). Reproduced from Knight SR, Russell NK, Barcena L et al. Mycophenolate mofetil decreases acute rejection and may improve graft survival in renal transplant recipients when compared with azathioprine: a systematic review. Transplantation 2009; 87(6):785–94. With permission from Wolters Kluwer Health. The early studies analysing the efficacy of sirolimus for maintenance therapy in kidney transplantation were done with sirolimus utilised in combination with prednisone and ciclosporin. The comparison groups received ciclosporin and prednisone plus azathioprine,17 or ciclosporin and prednisone plus placebo.18 Both of these large studies demonstrated improved efficacy of sirolimus compared to azathioprine or placebo, when used in combination with prednisone and ciclosporin (less BPAR, no difference in allograft or patient survival). Despite this improvement in acute rejection episodes, both studies demonstrated that the sirolimus-treated patients had higher serum creatinine levels at 6 months and 1 year. This raised concern about the combination of sirolimus with full-dose CNI as the cause of worsening allograft function. Other studies have confirmed this apparent negative synergistic nephrotoxic effect of sirolimus with full-dose CNIs.19–21 Furthermore, the de novo use of sirolimus has been associated with lymphocele formation, prolonged recovery from delayed graft function and poor wound healing.22–24 Given these issues, several recent trials have attempted to initiate maintenance therapy with a CNI and subsequently switch to an mTOR inhibitor in an attempt to minimise the long-term nephrotoxicity of CNIs or the combination of calcineurin and mTOR inhibitors. The most recent is the CONVERT trial, a large, randomised trial in which recipients of renal allografts who were 6–120 months post-transplant and receiving tacrolimus or ciclosporin were randomly assigned to continue CNI or convert from CNI to sirolimus.25 At 12 and 24 months there were no significant treatment differences in glomerular filtration rate (GFR) in the baseline GFR of more than 40 mL/min stratum. On-therapy analysis of this cohort showed significantly higher GFR at 12 and 24 months after sirolimus conversion. Rates of BPAR, graft survival and patient survival were similar between groups. Median urinary protein-to-creatinine ratios were similar at baseline but increased significantly after sirolimus conversion. Post hoc analysis identified a subgroup with baseline GFR more than 40 mL/min and urine protein-to-creatinine ratio less than or equal to 0.11 whose risk–benefit profile was more favourable after conversion than that for the overall sirolimus conversion cohort. The ZEUS trial was a large, randomised trial in which recipients of renal allografts were induced with basiliximab (see below) and maintained on three-drug therapy with ciclosporin, prednisone and MMF.26 At 4.5 months post-transplant, patients were randomised to either continue ciclosporin or switch to everolimus. At 12 months, patients who received everolimus had improved GFRs. However, there was a higher incidence of acute rejection, hyperlipidaemia, anaemia and proteinuria. The Spare-the-Nephron trial was a large, multicentre, randomised trial in which patients were initially maintained on MMF and a CNI and then randomised to either stay on the MMF/CNI combination or switch to MMF plus sirolimus 30–180 days post-transplant.27 At 12 months, the sirolimus group had significantly better GFRs; however, at 24 months this difference was no longer statistically significant. Also, there was a non-significant trend towards a lower rate of rejection in the sirolimus group. New biological agents currently in development have the potential to alter the model of immunosuppression drug design and delivery for organ transplantation. In general, biologicals and antibodies have been relegated to perioperative induction and the treatment of rejection due to their side-effects or an immune response to the agents themselves, limiting long-term efficacy. However, novel biologicals are now being developed for use as maintenance immunosuppression therapy. By selecting specific cell surface targets, new antibodies demonstrate remarkable specificity. This enhanced specificity is ideally suited for long-term immunosuppression as it may alleviate the untoward toxicity seen with daily agents such as CNIs. Technology such as protein humanisation as well as the ability to eliminate long-term immunogenicity has made antibody and fusion protein receptor therapy a present-day reality.28 The transition, for biological agents, from short-term perioperative induction therapy to chronic maintenance immunosuppression was driven by a desire to eliminate CNIs and their toxicities. Furthermore, the development costs of a biological agent can only be justified when coupled with planned chronic use. The challenge, of course, is to demonstrate the safety of inhibiting a particular pathway with a biological over long periods of time. The prototype of such an endeavour is belatacept. E-ATG, a depletional agent, was first studied in renal transplantation as part of an induction regimen.29 This important study was an open-label trial that enrolled 358 renal transplant recipients. Patients were randomised to receive E-ATG for 14 days at the time of transplant in addition to standard immunosuppressive therapy, which at the time included azathioprine and prednisone (n = 183), versus standard immunosuppression without E-ATG (n = 175). E-ATG was dosed between 10 and 30 mg/kg/day. The outcomes evaluated were time to first rejection episode, steroid dosage requirements, graft and patient survival. A total of four lots of E-ATG were tested. Results within each lot were analysed separately and the data were also pooled to obtain an overall impression. In the pooled data analysis E-ATG delayed the onset of the first rejection episode during the prescribed treatment period (2 weeks). Concurrently, fewer i.v. corticosteroids were required, but the steroid dosage requirement then rebounded in the 2 weeks after the end of the prescribed treatment period. E-ATG did not significantly improve the proportion of patients alive with functioning grafts 6 months after transplantation, except in one of the four lots. Later, Shield et al. published their experience using E-ATG for the treatment of acute rejection rather than as part of an induction regimen.30 A total of 20 patients with rejection were randomised to receive E-ATG 15 mg/kg for 14 days or methylprednisolone 1000 mg i.v. daily for up to 5 days. Maintenance immunosuppression consisted of azathioprine and prednisone in both groups. Both therapies successfully reversed rejection episodes in these 20 patients, with E-ATG reversing rejection on average in 3 days and methylprednisolone in 6 days. Although underpowered to detect true differences in efficacy between these two regimens, these early data were able to demonstrate that E-ATG was at least as effective as methylprednisolone in reversing rejections and E-ATG may achieve that goal more quickly than methylprednisolone. The intriguing data of Shield et al. prompted an evaluation of E-ATG for the treatment of steroid-resistant rejections in kidney transplant recipients. Hardy et al. evaluated 10 patients who did not respond to their usual treatment of acute rejection, which included methylprednisolone 15 mg/kg/day (not to exceed 750 mg) plus graft irradiation on days 1, 3 and 5 after rejection diagnosis.31 After failure of usual therapy to reverse acute rejection these 10 patients were treated with E-ATG (15 mg/kg/day for 21 days). Their response to this therapy was then described and compared to that of 10 other patients who were treated with and responded to the typical rejection protocol (control group). Outcomes of interest were return of serum creatinine to baseline, frequency of other rejection episodes following E-ATG treatment, and 1-year patient and graft survival. Seven of the 10 patients in the E-ATG group experienced a return of creatinine to baseline compare to only three of the 10 patients in the control group. Two patients in the E-ATG group experienced a second rejection episode within 1 year and no patient in the E-ATG group experienced a third rejection episode. In contrast, four patients in the control group experienced a second rejection episode and two of these patients experienced rejection a third time within 1 year. Serum creatinine concentrations also were lower in the E-ATG group compared to the control group 1 year later. Graft failure appeared to occur equally among the two groups, with five and four grafts lost in the control and E-ATG groups, respectively, over the 1-year follow-up. There were no deaths in either group during the follow-up period. Later, this same group published their experience with 12 additional patients receiving E-ATG for the treatment of a first acute rejection episode compared to 20 control patients receiving the standard protocol for their first acute rejection.32 Graft survival at 1 year was 73% and 46.6% for patients treated with E-ATG and standard therapy, respectively. Mortality was 3% and 20% in the E-ATG and standard therapy groups, respectively. Therefore, the data suggested that E-ATG was superior to standard therapy for graft and patient survival for the treatment of a first acute rejection episode. R-ATG was approved in the USA for the treatment of acute rejection in renal transplant recipients in 1999. R-ATG is extensively used off-label as induction immunosuppression given at the time of transplantation. R-ATG is a polyclonal IgG antibody, produced by immunising rabbits with human T lymphocytes. R-ATG is comprised of multiple different antibodies directed against various T- and B-lymphocyte surface antigens, such as CD2, CD3, CD4, CD8, CD11a, CD18, CD25, human leucocyte antigen (HLA)-DR and HLA class I. R-ATG exerts its antirejection effects by clearing T lymphocytes from peripheral blood and modulation of T-lymphocyte activity. When used for the treatment of acute rejection R-ATG is usually administered in doses of 1.5 mg/kg i.v. daily for 7–14 days. Primary side-effects are related to an infusion syndrome consisting of various signs and symptoms, including fever, chills, rigors, headache, shortness of breath, and hyper- or hypotension. These side-effects can be minimised with the addition of corticosteroid, acetaminophen and antihistamine premedication. Additionally, haematological side-effects can occur; these include leucopenia and thrombocytopenia. These may persist for several weeks after the last infusion of R-ATG. Complete blood counts should be monitored closely during and after therapy. The manufacturer of R-ATG recommends that the dose should be halved if the white blood cell count (WBC) drops to between 2000 and 3000 cells/mm3 or if the platelet count falls to between 50 000 and 75 000 cells/mm3. The manufacturer also recommends considering stopping R-ATG treatment if the WBC falls below 2000 cells/mm3 or the platelet count falls below 50 000 cells/mm3. Many centres administer R-ATG according to the absolute T-cell count with daily monitoring of this parameter, as individual patient response to the same dose regimen can be very variable.33 R-ATG was studied in a double-blind, randomised, multicentre trial compared to E-ATG for the treatment of acute rejection following kidney transplantation.34 A total of 163 patients were enrolled at 25 transplant centres in the USA. Patients were randomised to receive R-ATG 1.5 mg/kg/day versus E-ATG 15 mg/kg/day, each for a total of 7–14 days. With regard to the primary end-point, reversal of acute rejection rate, R-ATG was superior to E-ATG (88% vs. 76%; P = 0.027). Day 30 graft survival (R-ATG 94% and E-ATG 90%; P = 0.17), day 30 serum creatinine levels as a percentage of baseline (R-ATG 72% and E-ATG 80%; P = 0.43) and improvement in post-treatment biopsy results (R-ATG 65% and E-ATG 50%; P = 0.15) were not statistically different.T-cell depletion was maintained more effectively with R-ATG compared to E-ATG both at the end of therapy (P = 0.001) and at day 30 (P = 0.016). Recurrent rejection at 90 days after therapy occurred less frequently with R-ATG (17%) compared to E-ATG (36%; P = 0.011). A similar incidence of adverse events, post-therapy infections, and 1-year patient and graft survival rates was noted in both treatment groups. Overall, R-ATG was found to be superior to E-ATG in reversing acute rejection and preventing recurrent rejection after treatment in renal transplant recipients. R-ATG has also gained common off-label use as an induction agent. R-ATG has been evaluated for this purpose in a randomised, double-blind, single-centre trial versus E-ATG with initial results published in 1999 and 10-year follow-up data published in 2008.35 The primary end-point was to compare the safety and efficacy of R-ATG to E-ATG as an induction agent in renal transplant recipients. A total of 72 kidney transplant recipients were randomised 2:1 in a double-blinded fashion to receive R-ATG (n = 48) at 1.5 mg/kg i.v. or E-ATG (n = 24) at 15 mg/kg i.v., intraoperatively, then daily for at least 6 days. By 1 year after transplantation, 4% of R-ATG-treated patients experienced acute rejection compared to 25% of E-ATG-treated patients (P = 0.014). Patient survival was not different between the two groups but the composite end-point of freedom from death, graft loss or rejection (the ‘event-free survival’) was superior with R-ATG (94%) compared with E-ATG (63%; P = 0.0005). Fewer adverse events occurred with R-ATG but leucopenia was more common with R-ATG than with E-ATG (56% vs. 4%, respectively; P < 0.0001) during induction. The incidence of cytomegalovirus (CMV) disease was less with R-ATG than with E-ATG at 6 months (10% vs. 33%, respectively; P = 0.025). At 5 years the event-free survival was 73% and 33% for R-ATG and E-ATG, respectively (P = 0.047). Freedom from rejection at 5 years was 92% and 66% for R-ATG and E-ATG, respectively (P = 0.007). No additional cases of CMV disease occurred in either group; however, there were two cases of post-transplant lymphoproliferative disorder (PTLD) with the E-ATG group and none in the R-ATG group. At 10 years the event-free survival was higher with R-ATG than with E-ATG (48% vs. 29%, respectively; P = 0.011). Patient and graft survival was 75% and 48%, respectively, for R-ATG-treated patients, and 67% and 50%, respectively, for E-ATG-treated patients. However, the incidence of acute rejection was lower in R-ATG-treated patients compared to E-ATG-treated patients (11% vs. 42%, respectively; P = 0.004). There were no new cases of PTLD or CMV in either group compared to the 5-year results.
Immunosuppression with the kidney as paradigm
Introduction
Calcineurin inhibitors
Tacrolimus
Antimetabolites
mTOR inhibitors
Biological agents
Depleting antibodies
Equine antithymocyte globulin
Rabbit antithymocyte globulin
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Immunosuppression with the kidney as paradigm
Only gold members can continue reading. Log In or Register to continue




