Imaging of the Surgically Altered Breast
Erini Makariou
Anousheh Sayah
Introduction
Over the past few decades the use of breast imaging has increased, with more emphasis being placed on ultrasound (US) and magnetic resonance imaging (MRI) than ever before. While mammography remains the front line in screening and first-time diagnosis, US and MRI allow a more accurate characterization of lesions and symptomatic findings. The number of breast cancer detections has increased, as have the instances of surgical breast interventions. In order to detect the abnormal changes in the breast, breast surgeons and breast imagers must be familiar with the normal findings of the postsurgical breast. The techniques of biopsy, implantation, and reconstruction have also evolved, changing the breast imaging findings as well. This chapter reviews the mammographic, sonographic, and MRI imaging of the breast after reduction, benign biopsy, conservation treatment, augmentation, and reduction, as well as autologous and nonautologous reconstruction.
Mammogram: Basic Principles
A review of the basics of mammography and mammographic interpretation is essential for understanding the imaging of the surgically altered breast. Mammography is performed on a dedicated, low-dose radiographic unit. The unit compresses the breast to allow separation of parenchymal structures, improve contrast and resolution, and minimize x-ray dose. Digital mammography units are quickly replacing film-screen units. Digital mammography captures x-ray information after it passes through the breast in a digital format—without a film—that can be viewed, edited, and transferred electronically to viewing stations and picture archiving and communications systems viewers.
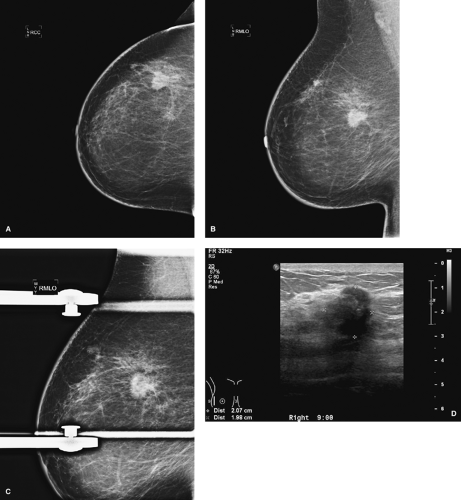
Standard mammographic views include craniocaudal (CC) and mediolateral (MLO) views (Fig. 3.1). In a craniocaudal view, the breast is compressed from top to bottom. In a MLO view, the breast is compressed obliquely side to side, parallel to the plane through the axilla and the pectoralis muscles. These complementary views allow three-dimensional localization of a lesion of interest. Radiopaque markers placed on the breast by the technologist indicate a skin lesion, palpable lesion, or visible scar.
A “screening” study is performed on an asymptomatic patient after the age of 40 years according to American College of Radiology guidelines (1). A “diagnostic” examination is performed when there are suspicious signs or symptoms or if the screening study was abnormal. During the diagnostic examination, the radiologist may request other specialized views, such as spot compression or magnification views, to further evaluate a suspicious area or lesion. A concomitant ultrasound exam may be performed to better characterize a lesion as solid or cystic. In all cases, old comparison views are critical in the diagnostic assessment and should be available for the radiologist’s review. Comparisons can demonstrate stability of a benign lesion or interval change in an active or malignant lesion. In many cases, comparisons will lower the number of radiographic images needed for the ultimate diagnosis. Signs of malignancy on mammography include spiculated lesions, architectural distortions, pleomorphic, casting, or linear calcifications, and/or asymmetry (Fig. 3.2).
Ultrasound: Basic Principles
Ultrasound is an essential component in the workup of a palpable or mammographically evident lesion. In breast US, sound waves are emitted into the breast, and an image is created by the returning sound waves received by the ultrasound detector. Advantages of breast ultrasound include the ability to better characterize the composition of breast parenchyma and to determine whether breast lesions are solid or cystic (Fig. 3.3). Ultrasound can be used for a targeted region of the breast. It is also highly specific, relatively inexpensive, and widely available. Sonography is ideal for guided drainage or biopsy of lesions. Poor visualization of microcalcifications, which are often a sign of malignancy, is the ultrasound’s main disadvantage. In addition, ultrasound can be labor intensive. However, whole-breast sonography is breaking ground as the first line of imaging (2,3).
Magnetic Resonance Imaging: Basic Principles
MRI of the breast has evolved into an important tool in breast imaging with increasing indications for its use. Contrast-enhanced breast MRI indications include (a) evaluation of the extension of known breast cancer, (b) detection of contralateral breast cancer in women with newly diagnosed breast cancer, (c) screening for women who are at high risk and/or positive for BRCA1 and BRCA2, (d) assessment of neoadjuvant chemotherapy response, (e) evaluation of chest wall invasion or mastectomy site for recurrence, and (f) detection of breast cancer in women with axillary metastases and a normal mammogram (4,5,6).
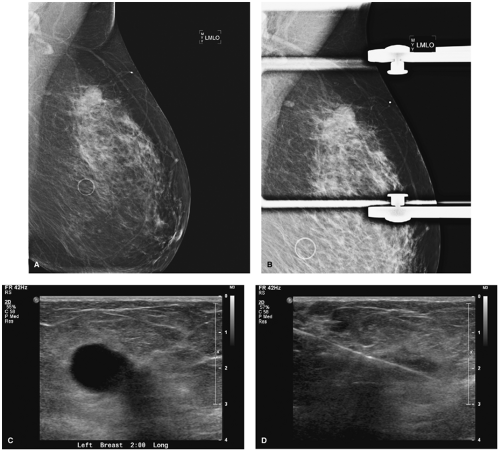
Morphologic characterization and dynamic enhancing pattern of a lesion play an important role in MRI interpretation. The high sensitivity of MRI but its not so high specificity create many false-positive results and callbacks for further evaluation, including second-look ultrasound studies and biopsies (Fig. 3.4). Despite this, MRI has very high negative predictive value for invasive ductal cancer in women with questionable findings on mammogram and ultrasound studies (7,8).
The newly recognized but rare side effect of MRI, nephrogenic sclerosing fibrosis, is related to intravenous administration
of MRI contrast agents. Special attention is needed for women with impaired renal function or renal failure (9).
of MRI contrast agents. Special attention is needed for women with impaired renal function or renal failure (9).
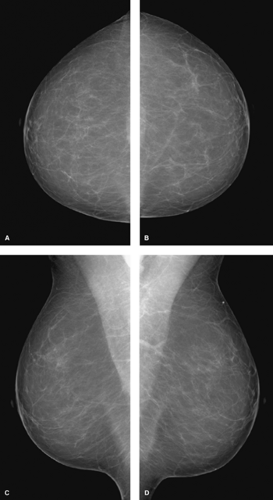 Figure 3.1. Screening mammogram. A: Craniocaudal view, right breast. B: Craniocaudal view, left breast. C: Mediolateral oblique view, right breast. D: Mediolateral oblique view, left breast. |
Preoperative Imaging
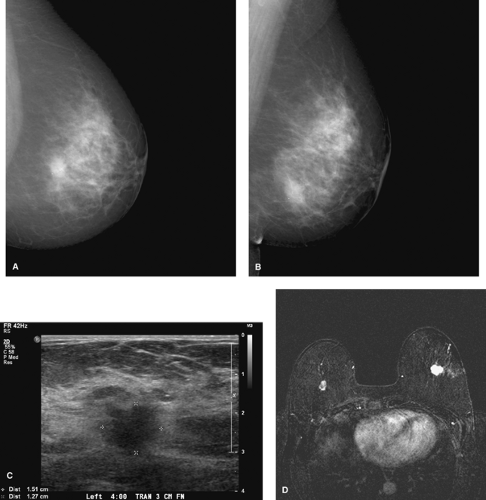
Preoperative imaging allowing better delineation of the extent of a tumor prior to definitive treatment is becoming a standard procedure. Preoperative imaging can change the case management approach in a significant number of cases: 18% using US and 11% to 30% using MRI (10,11,12). US and MRI are generally more sensitive than mammography for invasive carcinomas, especially in dense and heterogeneous breasts. MRI is commonly used for the detection of multicentric and multifocal diseases once cancer is diagnosed (Fig. 3.5). Most additional tumor foci (87%) are located in the same
quadrant as the original tumor (10). Axillary and internal mammary chain nodes can also be evaluated. Suspicious nodes lose the normal fatty hilum and may enhance more than other nodes (10).
quadrant as the original tumor (10). Axillary and internal mammary chain nodes can also be evaluated. Suspicious nodes lose the normal fatty hilum and may enhance more than other nodes (10).
Benign Biopsy Changes
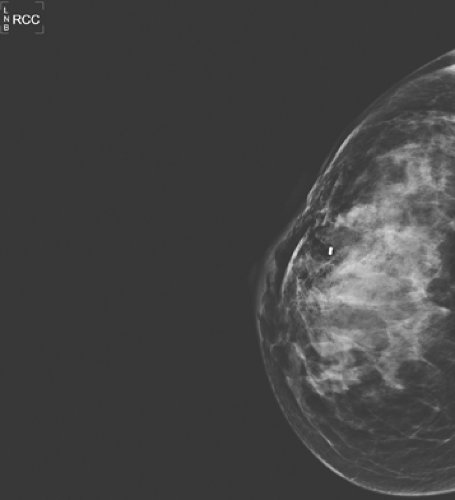
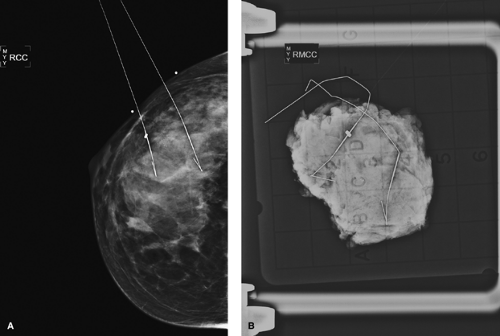
Breast biopsies may be performed in the radiology suite or in the operating room by a breast surgeon. Core biopsies are often performed with radiographic, sonographic, or MRI guidance under local anesthesia. An 8- to 14-gauge core needle either in a spring-loaded or vacuum-loaded device is used for sampling. In most cases, a localizing clip is placed in the biopsy site for future reference. A post–core biopsy mammogram is performed to verify the relationship of the clip to the biopsy bed and to serve as a new baseline.
Post–core biopsy mammographic changes are few and very uncommon. In cases of clip placement, a small metallic clip should be visible at the biopsy site. Clips may demonstrate some migration from the area of initial placement (13). In cases with postbiopsy parenchymal hemorrhage, a localized area of increased density corresponding to increased fluid and blood may be seen. Needle tracts may be noted as subtle, cylindrical mammographic densities in a projection parallel to the biopsy tract with 11-gauge needles. Fourteen-gauge needles have not been shown to produce any detectable abnormality (14). Following vacuum-assisted core biopsy, gas pockets and hematomas are common, but they resolve within 2 to 4 weeks (15) (Fig. 3.6). Overall, post–core
biopsy changes should not be recognized on mammography after approximately 6 months; any density after this period likely represents an abnormality and should be further evaluated (16).
biopsy changes should not be recognized on mammography after approximately 6 months; any density after this period likely represents an abnormality and should be further evaluated (16).
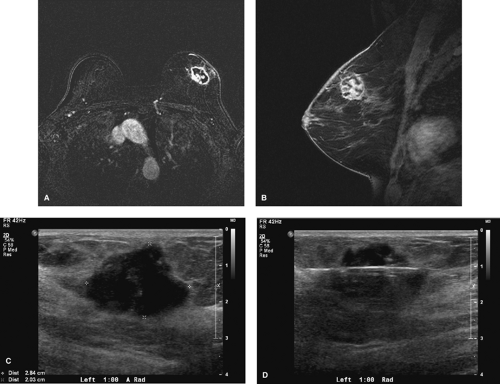 Figure 3.4. A: Axial magnetic resonance imaging, cancer. B: Sagittal magnetic resonance imaging, cancer. C: Ultrasound, second-look cancer. D: Ultrasound, biopsy cancer. |
Postexcisional breast biopsies, which involve the removal of a larger amount of breast tissue, demonstrate significantly more imaging findings. Fifty percent of benign excisional breast biopsies show some mammographic change (17). Postsurgical mammograms are ideally performed at about 3 to 6 months after surgery and serve as new baseline views. Benign pathologies, however, may not prompt postsurgical imaging unless there is a need to confirm successful removal of a lesion or evaluate for postoperative complications.
In a postsurgical mammogram, a thin metallic marker is placed on the surgical skin scar to indicate its location on the images. The most commonly viewed postexcisional change is architectural parenchymal distortion, followed by overlying focal skin thickening (17) (Fig. 3.7). These changes are seen mostly in the first 6 months after surgery (18). Other findings include calcifications or fat necrosis.
Occasionally, 6 or more months after the procedure (18), calcifications form in the postexcisional bed. Most are dystrophic in nature or associated with fat necrosis or oil cysts. Small irregular calcifications mimicking malignancy may also be seen and should be closely monitored for malignancy.
Specimen Imaging
Biopsy samples can be imaged radiographically or sonographically in order to verify the removal of the lesion or of calcifications or to assess surgical margins. The specimen x-ray is more sensitive than the in vivo mammogram due to increased resolution from the lack of surrounding attenuating breast tissue, increased penetration due to sample compression, decreased scatter radiation, lack of motion, and ability to use higher radiation doses (19). In fact, specimen views may show calcifications that were not seen before the biopsy (20) (Fig. 3.8).
Only lesions seen sonographically before biopsy are imaged sonographically after excision. MRI is not technically possible for specimen imaging. However, MRI needle localization specimens are often mammographically evaluated to show the integrity of the wire.
Imaging the Postreduction Mammoplasty Breast
Most changes after breast reduction are identified in the lower breast, with skin thickening and distorted architecture most commonly seen on mammography. The ducts may appear to converge lower than the nipple, since the nipple is often reimplanted superiorly. Due to the surgical trauma from reduction, common postsurgical findings can be seen, including fat necrosis, oil cysts, and epidermal inclusion cysts. On US or MRI, skin thickening is easily detected. However, in the absence of a focal lesion, the breast parenchyma will likely show no difference from the normal breast (21,22) (Fig. 3.9).
Imaging the Conservatively Treated Breast
Current widespread use of breast conservation therapy necessitates familiarity with postconservation imaging, most importantly to aid in detection of residual tumor or recurrence. Conservation treatment includes lumpectomy with or without axillary node dissection and/or radiation, tailored to each patient’s individual needs. Surveillance after conservation includes mammography, US, and/or MRI examination, in conjunction with physical examination.
Mammography continues to be the first line of imaging for screening of postlumpectomy patients, although sensitivity
is lower than in nontreated breasts due to confounding postoperative changes (23). Early postoperative mammography is performed to confirm the removal of the lesion, identify postoperative fluid collections, detect residual and recurrent cancers, and screen for metachronous cancers in either breast. Views are performed approximately 2 weeks after surgery, prior to radiation treatment, at a time when postsurgical pain and edema have lessened and hematomas have resolved. A wire marker is applied to the surgical scar to identify the surgical plane, and CC and MLO views are obtained. Compression spot magnification views of the lumpectomy site are performed to evaluate for residual calcifications, especially if calcifications were present in the original tumor.
is lower than in nontreated breasts due to confounding postoperative changes (23). Early postoperative mammography is performed to confirm the removal of the lesion, identify postoperative fluid collections, detect residual and recurrent cancers, and screen for metachronous cancers in either breast. Views are performed approximately 2 weeks after surgery, prior to radiation treatment, at a time when postsurgical pain and edema have lessened and hematomas have resolved. A wire marker is applied to the surgical scar to identify the surgical plane, and CC and MLO views are obtained. Compression spot magnification views of the lumpectomy site are performed to evaluate for residual calcifications, especially if calcifications were present in the original tumor.
In order to establish a postoperative baseline, a unilateral examination is also performed 6 months after completion of conservative therapy. Annual bilateral mammograms are performed thereafter to monitor for disease presence. Serial 6-monthly exams may be performed for several years postdiagnosis if clinically warranted.
Changes are most marked in the first year after surgery, peaking at 6 to 12 months (24). During the following 5 years, postsurgical findings (with the exception of calcifications) decrease in prominence (25). Findings are usually multiple and include architectural distortion (82%), increased regional density (79%), skin thickening (54%), and calcifications (3%). Lipid cysts, hematomas, and seromas may also be present (25).
Postlumpectomy parenchymal scarring is usually identified as architectural distortion and increased parenchymal density. Postlumpectomy scarring is of relatively low density, interspersed with radiolucent areas that represent entrapped fat, which can be helpful in distinguishing it from malignancy.
Postoperative fluid collections are also culprits of increased regional breast density, seen in 50% of patients at 4 weeks and
25% of patients at 6 months after surgery (26). These fluid collections can appear as a mass, but they should gradually decrease in size, stabilize, or completely resolve. As opposed to benign excisions, dead space is purposely left behind in malignant excisions to allow fluid to fill in for better cosmesis (Fig. 3.10).
25% of patients at 6 months after surgery (26). These fluid collections can appear as a mass, but they should gradually decrease in size, stabilize, or completely resolve. As opposed to benign excisions, dead space is purposely left behind in malignant excisions to allow fluid to fill in for better cosmesis (Fig. 3.10).
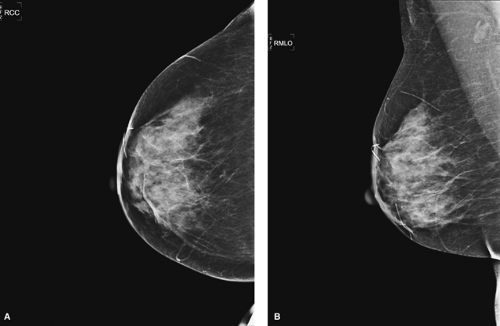 Figure 3.7. A: Craniocaudal view of right breast, postexcision. B: Mediolateral oblique view of right breast, postexcision. |
Benign calcifications after conservative therapy include oil cysts, dystrophic calcifications, and calcified suture material. Calcifications due to fat necrosis typically develop at or near the lumpectomy site, usually 2 years after treatment (27).
The rate of tumor recurrence after lumpectomy and radiation, usually found 2 or more years after treatment (28,29,30,31), is 5% to 10% at 5 years and 10% to 16% at 10 years. Mammography has been shown to detect two thirds of these cases (32). From 33% to 50% of local recurrences are detected on mammography prior to examination and are less likely to be invasive than those found physically (33,34).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree