Fig. 5.1
Classification of male hypogonadism as a function of age of onset and patient’s phenotype. Schematic prevalence in male population is also shown. Size of ellipsis reflects on abscissa (log scale): age of onset and on ordinates (log scale): incidence (right axis) or female to male phenotype (left axis, arbitrary unit). VEOH: very early onset hypogonadism, i.e., starting during fetal life for absence of testosterone formation or activity (e.g., complete androgen insensitivity or Morris’ Syndrome, blue ellipsis) or impaired secretion or activity of GnRH (e.g., Kallmann’s syndrome or mutation in GPR54 and GnRH receptor, red ellipsis). EOH early onset hypogonadism (i.e., peri-pubertal onset, such as in Klinefelter’s syndrome, green ellipsis). LOH late onset hypogonadism, i.e., in adulthood or aging (brown ellipsis) (Adapted from Ref. [9])
Table 5.1
More and less specific clinical symptoms and signs associated with hypogonadism
Symptoms | Clinical signs | |
|---|---|---|
More specific | Sexual Reduced sexual desire (libido) Erectile dysfunction Decreased spontaneous erections Physical Decreased vigorous activity Difficulty walking >1 km Inability to bend Psychological Sadness Loss of energy Fatigue | Increased body fat, body mass index Very small (especially <5 ml) or shrinking testes Decreased prostate size |
Less specific | Sexual Decreased frequency of intercourse Decreased autoeroticism Delayed ejaculation Physical Hot flushes, sweats Decreased energy, motivation, initiative, and self-confidence Reduced muscle bulk and strength Diminished physical or work performance Psychological Poor concentration and memory Sleep disturbance, increased sleepiness | Loss of body (axillary and pubic) hair, reduced shaving Gynecomastia |
5.3 Definition of Late Onset Hypogonadism
Different T thresholds have been proposed for the biochemical definition of low T [3–5]. According to major international guidelines, T substitution has to be offered to symptomatic individuals when circulating total T is below 8 nmol/L (231 ng/dL). In addition, there is also general agreement that a total T level above 12 nmol/L (346 ng/dL) does not require substitution. When total T is repetitively >8 and <12 nmol/L, in the presence of typical hypogonadal symptoms (as listed before), a T treatment trial might be considered [3–5]. In addition, it should be recognized that a pathological valuate of T must be confirmed in a second sample before prescribing T replacement therapy (TRT) [3–5].
5.4 LOH and Obesity, Clinical Evidence
The association between LOH, obesity, MetS, insulin resistance, and T2DM is well known [5–7, 11–14]. Accordingly, in two independent meta-analyses of the available evidence, we reported that subjects with MetS and T2DM have significantly reduced T levels (about 3 nmol/l lower) [15, 16]. Zumoff et al. [17] and ourselves [18] previously showed that SHBG-bound and unbound testosterone levels decreased in obese males in proportion to the degree of their obesity, even after adjustment for obesity-related conditions (see also Fig. 5.2). In addition, data from morbidly obese men indicate that LH levels and pulse amplitude were attenuated when compared to normal weight controls [19, 20]. These observations support the concept of a true obesity-associated HH. The specific pathogenetic mechanisms linking LOH with insulin resistance, MetS, and T2DM appear to be complex and often multi-directional (see Fig. 5.3). Obesity is characterized by a relative abundance of estrogens since P450 aromatase is highly expressed by fat tissue. The increased amount of estrogen levels, might, in turn, have a negative effect on both the hypothalamus and the pituitary, leading to decreased LH secretion [6, 7]. Accordingly, it has been reported that the use of the aromatase inhibitor letrozole can restore T levels and increase LH levels in severely obese hypogonadal men [21]. In line with this view, we now show that body weight loss, obtained either through lifestyle or bariatric intervention, is associated to a fall in estrogen levels and with a rise in gonadotropins and T [6]. However, other fat-associated factors, besides estrogens, have been proposed as a link between obesity and reproductive axis disorders: a series of adipokines and among them, the most extensively studied are leptin, ghrelin, and adiponectin ([22] see also Fig. 5.3).
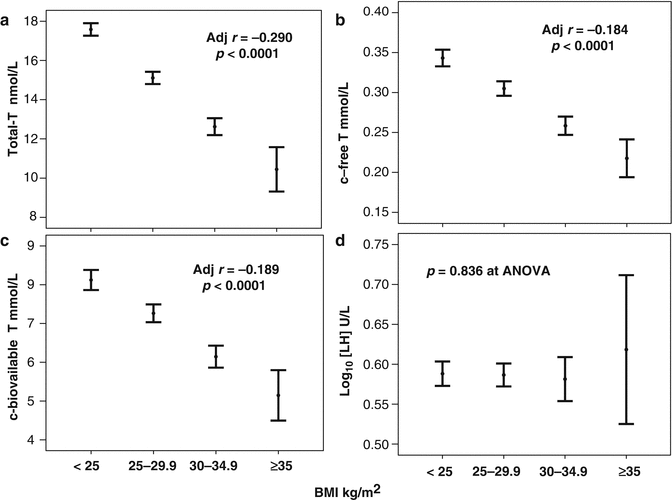
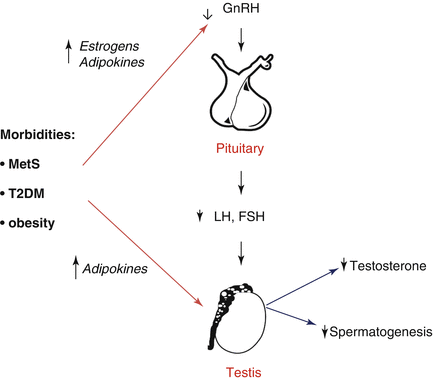

Fig. 5.2
Testosterone (T; panels a–c) and luteinizing hormone (LH, panel d) levels as a function of obesity classification. T testosterone, c-free-T and c-bioavailable-T = calculated free and bioavailable testosterone according to Vermeulen formula. BMI body mass index. Data are expressed as mean [95 % confidence interval]. Data are derived from a consecutive non-selected series of men (mean age = 51.3 ± 13.3 years) attending our Sexual Medicine & Andrology Clinic for sexual dysfunction between 2000 and 2013 (unpublished). The inset indicates the age adjusted data. BMI was considered as a continuous value

Fig. 5.3
Proposed interactions between increased visceral fat and hypogonadism. MetS metabolic syndrome, T2DM type 2 diabetes mellitus, LH luteinizing hormone, FSH follicle stimulating hormone, GnRH gonadotropin-releasing hormone, T testosterone
5.5 LOH and Obesity: Experimental Studies
The contribution of the different metabolic derangements on the related condition of HH has been recently investigated in an animal model of high fat diet (HFD)-induced MetS [23]. The model, established in adult male rabbits fed a HFD for 3 months, has been largely characterized by our group [24–30] and recapitulates the human phenotype, including visceral obesity, hypertension, dyslipidemia, and glucose intolerance. Moreover, as in humans, HFD rabbits exhibit an overt HH, with low plasma levels of testosterone, luteinizing hormone (LH), and follicle-stimulating hormone (FSH), and reduced androgen-dependent organ weight. In this experimental model, sex hormone imbalance was associated with MetS severity, since T decreased and estrogen increased as a function of the number of MetS components [23]. Also, gonadotropin plasma levels were negatively associated with MetS and, among MetS factors, hyperglycemia and hypercholesterolemia resulted as being the major determinants for the negative association with LH levels, while the dyslipidemic component (high cholesterol and triglycerides) appeared to be associated with FSH reduction [23]. Moreover, hormonal alterations also correlated with glucose intolerance severity. Since the pituitary gonadotropins are positively controlled by GnRH and a reduced content of GnRH neurons was demonstrated in the hypothalamus from HFD rabbits [24], the role of HFD-related alterations in the hypothalamic area in determining the documented dysfunctions of the gonadotropic axis have been extensively investigated. A close association of MetS – and in particular the related altered glucose and lipid metabolism – has been identified with peculiar hypothalamic alterations, including increased expression of the glucose transporter 4 (GLUT4) and inflammation. Indeed, HFD determined a low-grade inflammation in the hypothalamus, significantly inducing microglial activation and interleukin-6 (IL-6) expression. Interestingly, all these hypothalamic derangements were, in turn, associated with LH and FSH reduction, and occurred in the preoptic area of the hypothalamus, lining the third ventricle, where GnRH neurons reside. Accordingly, the same hypothalamic area was characterized by a reduced immunopositivity not only for GnRH [24], but also for Kisspeptin-1 receptor (KISS1R; 23), which along with its natural ligand kisspeptin represents the most characterized system mediating, at central level, the effects of a range of metabolic inputs known to regulate GnRH secretion [31, 32]. However, a not fully clarified issue is whether metabolic alterations act directly on GnRH neurons or are mediated by other integrating factors. Indeed, recent findings demonstrated that a subpopulation of GnRH neurons projects dendrites in regions outside the blood–brain barrier, where they may directly sense molecules circulating in the bloodstream [33]. Hence, the range of factors that are integrated by GnRH neurons for the control of the GnRH/gonadotropin secretion could be extended. Using a well characterized cellular model, we identified a direct inhibitory action of increasing glucose concentrations on human fetal GnRH-secreting neurons, the FNC-B4 cells [34–37], thus opening new mechanistic insights into the direct metabolic control of GnRH release [38]. FNC-B4 cells express glucose transporters (GLUT1, GLUT3, and GLUT4) and may respond to changes in glucose concentrations. Exposing FNC-B4 cells to high glucose significantly reduced the expression not only of GnRH but also of genes relevant for GnRH neuron function, such as KISS1R and leptin receptor. Even if obtained in vitro, these findings support the idea of a direct, deleterious contribution of hyperglycemia on human GnRH neurons, thus improving our understanding about the pathogenic mechanisms linking HH to metabolic disorders. Overall, in vitro and in vivo experimental studies indicate that metabolic derangements may activate proinflammatory pathways within the hypothalamus, thus compromising a key brain area involved in the control of reproduction. In agreement with this possibility, in vivo treatment with obeticholic acid, a drug that ameliorates glucose metabolism in the rabbit MetS model [24, 29], not only reverted all the HFD-induced hypothalamic alterations – including GLUT4 induction and inflammatory response – but also increased GnRH mRNA expression [23].
5.6 Testosterone Therapy and Obesity
In obese individuals, several studies have demonstrated that intense lifestyle intervention, along with nutritional counseling and physical activity, is able to reduce weight loss and to conjointly raise T levels. A recent meta-analysis, in fact, showed that weight loss is associated with an increase of bound and unbound T levels, along with gonadotropin levels, and that the final effect is directly related to the amount of body mass index (BMI) reduction [6]. Accordingly, the analysis of longitudinal data of the European Male Aging Study demonstrated that weight loss was associated with a proportional increase – and weight gain with a proportional decrease – in total T and sex hormone binding globulin [39




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








