Fig. 20.1
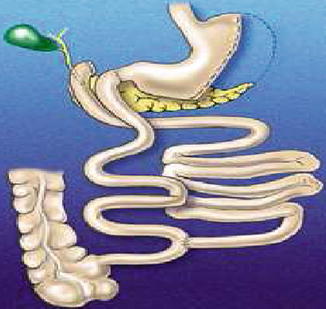
Scopinaro’s biliopancreatic diversion, 1979
Fig. 20.2
Intestinal measurement in open surgery
Fig. 20.3
Intestinal measurement in laparoscopic surgery
In summary, except for the unavoidable reduction of calcium and iron, due to the duodenal-jejunal bypass, and of liposoluble vitamins, consequent to the reduced fat absorption, assuming that simple sugars are fully absorbed by the small bowel in the alimentary stream, the absorption of all the other high-caloric nutrients in the standard BPD essentially depends on the volume of the gastric remnant (which, if too small, can cause a rapid gastric emptying and intestinal transit, thus reducing absorption capacity) and on the length of the intestinal limbs. The BPL should be long enough to allow complete digestion-absorption of pancreatic enzymes; the AL plus the CL should be long enough to guarantee, together with the colon, a protein absorption sufficient to counteract the endogenous nitrogen loss, as well as a sufficient water, electrolyte, and water-soluble vitamin absorption, still not allowing an excessive polysaccharide digestion-absorption; CL should be short enough to reduce as much as possible fat absorption, still allowing a bile salt absorption sufficient not to cause bile acid diarrhea. Incidentally, the loss of bile salt into the colon, together with the lack of gallbladder contraction, causes the constant presence of a lithogenic bile, which in turn would cause gallstone formation in the almost totality of operated patients, if a prophylactic cholecystectomy were not added to the operation in all cases.
It is very important to remember that the characteristics of very long term individual and interindividual weight maintenance, together with accurate studies of energy, fat, and protein intestinal absorption [4], and of postoperative changes of resting energy expenditure [2], lead to the hypothesis, subsequently verified by an overfeeding study [2], that a maximum daily intestinal absorption capacity exists for each standard BPD individual for fat and starch, while simple sugars are entirely absorbed, and protein absorption is a percent of the intake, corresponding to about 70 % [4]. In other words, the original intestinal lengths and gastric volume being equal, the interindividual variability of the weight of stabilization in BPD subjects is accounted for by interindividual differences of: (1) the original energy absorption capacity per unit of intestinal surface; (2) the entity of postoperative intestinal adaptation phenomena; (3) the intestinal transit time (which, in addition to gastric volume, can be influenced by the intake of fluids); (4) the simple sugar and protein intake; (5) the number of meals per day and the meal duration; (6) the body weight itself and the energy expenditure per unit of body mass. However, in each BPD individual the weight of stabilization cannot be modified by any increase or decrease of fat and starch intake, provided that the intake is greater than the maximum daily absorption capacity (also defined as “maximum transport threshold”).
Apart from the many overt modifications of the standard BPD, like preservation of the distal stomach [6] or of the entire stomach [7], or modifications of the intestinal limb lengths [8] (Figww. 20.4), and, more recently, the so-called duodenal switch [9, 10] (Fig. 20.5), which will be the object of another chapter of this book, once the BPD as a mechanism of action has been clearly described it is easy to recognize it in bariatric operations which have apparently nothing to do with BPD. The most obvious example is Roux-Y gastric bypass, where, even in its classic version with short BPL and AL [11–13] (Fig. 20.6), the meeting between food and biliopancreatic juices is delayed. In this operation it is generally assumed that the mechanism for calorie absorption reduction is ineffective, because the length of the CL is such that a complete calorie-rich aliment occurs. This is only partially true, as in many subjects with standard RYGB we have found a mild to moderate steatorrhea, in most cases confined to the first postoperative year. Anyway, it seems that the BPD mechanism is active, the threshold for intestinal fat absorption being exceeded, even if only minimally and temporarily, and that the initial weight reduction after RYGB is initially at least partially due to calorie malabsorption. The close similarity of RYGB to BPD is witnessed by the many surgeons who, with the very questionable aim of adding energy malabsorption to gastric restriction, use longer AL, or BPL, or both, thus originating operations called “distal GB” [14], or “long-limb GB” [15, 16], or even “very very long-limb GB” [17]. These operations, prompted by poor knowledge of the BPD mechanism, are very dangerous, because it was repeatedly demonstrated that to add malabsorption to gastric restriction is either just useless or even harmful for protein nutrition [14, 18].
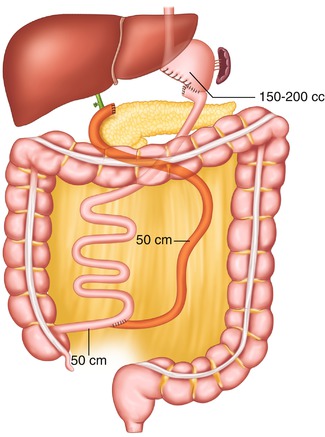
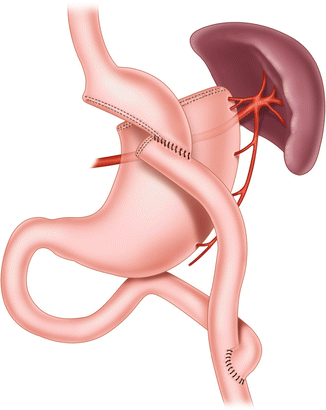

Fig. 20.4
Larrad’s biliopancreatic diversion

Fig. 20.6
Miller’s divided gastric bypass, 1979
Another evident case of misunderstanding is the so-called mini-gastric bypass or MGB, which should be more correctly and meaningfully named “single anastomosis GB” or “loop GB.” Pioneered by Rutledge [19], MGB consists of a GB where the pouch is created much longer than usual (to prevent esophageal reflux), and the Roux-Y anastomosis is replaced by a loop one, placed in all cases 200 cm distal to the ligament of Treitz, with the aim of sparing one enteroenterostomy. The operation was severely criticized in the USA, but it is being increasingly performed in many non-American countries, where the authors generally report the same good weight loss result as in the standard RYGB, but with less complications and shorter hospital stay. Interestingly, with the exception of Sanchez-Pernaute [20], who replaced the classic gastric pouch with a sleeve gastrectomy in order to avoid also gastric bile reflux (Fig. 20.7), none of the surgeons who perform MGB (including the originator) seems to have realized that the operation is a BPD, already published as such by Scopinaro in 1980 [21] as one of the possible versions of the standard BPD (Fig. 20.8), where no AL exists, as the gastric remnant directly empties into the common limb, which, also exerting the functions of the AL, determines the existence, the quality, and the entity of malabsorption, and consequently is the one that should be measured. As almost no surgeon measures the small bowel in condition of full reproducibility, that is fully stretched, in case of short small bowel (e.g., around 400 cm, which is not a very infrequent case), the 200 cm that Rutledge and the vast majority of the other authors measure distal to the ligament of Treitz, thus totally ignoring what is left distal to the GEA, could very well leave them with a common limb, whose length is not known to the surgeon, short enough to cause severe energy and protein malnutrition.
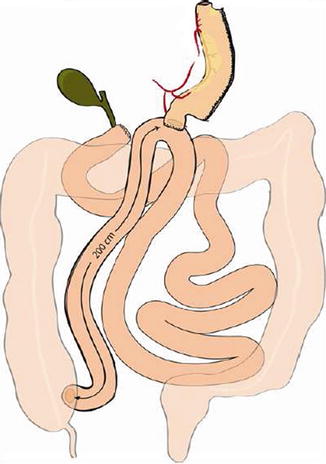
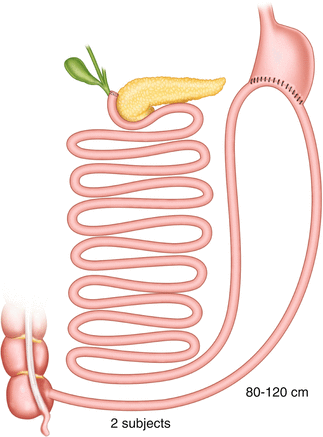

Fig. 20.7
Single anastomosis duodeno-ileal bypass with sleeve gastrectomy (SADI-S, 2010)

Fig. 20.8
Scopinaro’s 1980 standard biliopancreatic diversion (bypass) with loop gastroenterostomy
An interesting example of unacknowledged BPD is the so-called endobarrier gastrointestinal liner [22], a short intraluminal sleeve anchored to the pylorus that delays the meeting of food with biliopancreatic juices, thus causing a certain degree of energy malabsorption and consequent weight loss. The device, 60–100 cm long, can be left in place only temporarily (generally 6 months), and it is used today to obtain a pre-surgical weight loss prior to bariatric surgery.
Evidently, the number of different types of BPD (laparoscopic or not) currently in use today is so huge that a description of each of them would be as impossible as useless. Therefore, we will only describe the technical aspects, the clinical management, and the outcome of the laparoscopic standard BPD (LSBPD) in our experience of little less than 300, and as reported by the few bariatric surgeons who used the same model of operation (laparoscopic or not).
20.2 Technical Aspects
The surgical technique [23] entails the use of five trocars, placed in the positions illustrated in Fig. 20.9. The patient is placed in a moderate anti-Trendelenburg position (20–25°). As a rule, we prefer to do first the cholecystectomy, which can be annoying due to the non standard trocar positions. The gastrocolic ligament is incised at about its midpoint and the dissection, which is carried out with a harmonic scalpel, is performed until the traction of the large curve allows for the mobilization of the gastric fundus, which implies that the avascular area is always sectioned. The first short vessel is rarely sectioned, exceptionally the second. The dissection of the lower greater curve and the sectioning of the right gastroepiploic and the right gastric vessels complete the isolation of the duodenum, which is divided with single application of endoGIA 60. The small curve is then isolated cranially with the ultrasound scissors, stopping 1 or 2 cm before the trunk of the left gastric artery (Fig. 20.10), and the gastric resection is carried out by repeated firing of endoGIA 60. To measure the gastric stump is very easy by filling with water (35 cm H2O) a condom which has been tied at the end of a nasogastric tube. Gastric volume should be measured until the surgeon becomes able to evaluate it by sight. Roughly, a gastric sectioning from the greater curve, moderately stretched, at approximately 15 cm from the cardias (Fig. 20.11) to the lesser curve at 5 cm from the cardias corresponds to a gastric volume of about 300 mL (Fig. 20.12). A distance of 20 cm along the greater curve corresponds to a volume of about 400 mL. This applies both to the laparoscopic and the open technique, as it was demonstrated by a check done in all cases of conversion (which, being due to problems with the GEA construction, was generally done after division of the stomach) at the beginning of our experience with the laparoscopic technique, when we started measuring again the gastric volume in order to reacquire the ability the gastric stump [23].
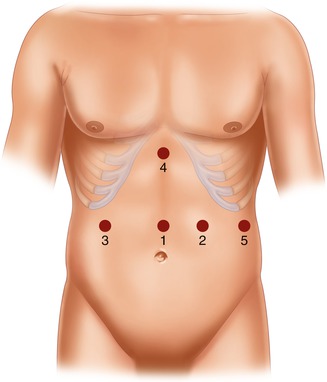
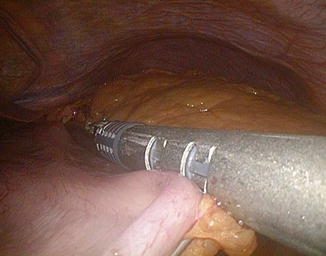
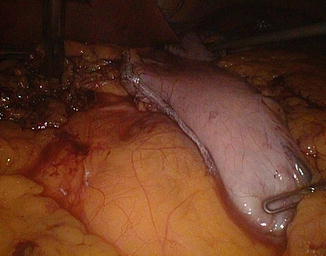

Fig. 20.9
Trocar position in laparoscopic standard BPD. 1: supraumbilical (10–12 mm), on the midline, 3–4 cm above the superior margin of the umbilicus; 2: left hypondriac (10–12 mm), along the left midclavicular line, about 6 cm below the costal margin; 3: right hypondriac (10–12 mm), along the right midclavicular line, about 6 cm below the costal margin; 4: xiphoid (10–12 mm), on the midline, 3 cm below the xiphoid; 5: left subcostal (5 mm), on the left costal margin, along the left middle axillary line

Fig. 20.10
Left gastric vessels

Fig. 20.11
Gastric measurement along the greater curve

Fig. 20.12
Gastric stump of approximately 300 mL
The patient is then placed in a slightly Trendelenburg position (about 10°). The small bowel is measured backward from the cecum, fully stretched (Fig. 20.3), using two forceps marked at 10 cm in alternating movements. A mark is left at 50 cm, the ileum is divided at 300 cm by using an endoGIA 45, and the mesentery is sectioned in depth with the ultrasonic scissors, taking care to proceed perpendicularly to its root (Fig. 20.13). The EEA is fashioned with a latero-lateral technique, with an endoGIA 45 through two small enterotomies made by the harmonic scalpel. The conjoined defect is closed with a manual running seromuscular suture.
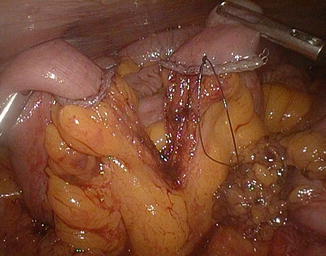

Fig. 20.13
Ileal division
The left angle of the gastric stump is then identified, grasped, and pulled into the submesocolic space through an incision performed in the transverse mesocolon over the ligament of Treitz (Fig. 20.14). The distal intestinal stump is identified, checked for possible torsion, and perforated with the ultrasonic scissors at a distance from the suture line equal to the operative length of the endoGIA 45. The latter is used to perform a latero-lateral isoperistaltic GEA on the posterior wall of the stomach, as close as possible to the distal angle and at midway between the suture line and the greater curve (Fig. 20.15), with manual closure of the conjoined defect. After the leak test with methylene blue introduced through the nasogastric tube, the GEA is anchored by two stitches to the mesocolic rent, in order to avoid intestinal kinkings and internal hernias (Fig. 20.16). We always close the distal mesenteric defect and never the proximal one (Patterson).
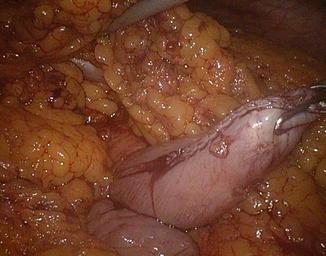
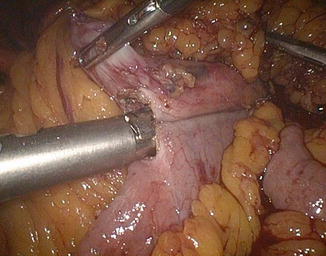
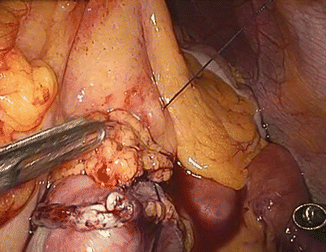

Fig. 20.14
Gastric stump attraction through the mesocolic rent

Fig. 20.15
Gastroenteroanastomosis

Fig. 20.16
Fixation of the GEA to the mesocolic rent
A liver wedge biopsy is always obtained. The last maneuver, as in open surgery, is the final intestinal check, starting from the ileocecal valve, with the surgeon following the AL and the first aid the BPL. It takes time, but it is the only way to make sure that no 360° intestinal torsion exists. As an alternative, the two mesenteric edges are carefully inspected from the anastomoses to the start of the mesenteric division.
The distal stomach, as well as the gallbladder, is extracted through the supraumbilical opening, which sometimes needs to be widened. Two drains are left in the Douglas pouch and under the liver. The trocars are removed under visual control. The fascial defect is sutured in the supraumbilical hole. The others are left as they are, with simple skin suturing.
As to the possible modifications of gastric volume and intestinal lengths, being similar all the other conditions, a larger gastric remnant generally, but not necessarily, results in a higher weight of stabilization, and certainly in a lower risk of protein malnutrition. It can be used precautionally in patients with lower compliance, never forgetting that low compliance should be considered in principle a contraindication to BPD, or in case of low BMI type 2 diabetic or hypercholesterolemic patients, when the aim of the operation is the exploitation of the specific actions for those conditions, with little or no weight loss (see below). On the contrary, a smaller gastric volume results in a more rapid weight loss with lower stabilization weight. The lower acceptable limit for gastric volume is about 300 mL (Fig. 20.12), as a smaller stomach has proven, in our [2, 18, 24] and in other authors’ experience [14], to entail a great risk of energy-protein malnutrition (PEM).
It is very important to remember that the small bowel is to be measured fully stretched, midway between the mesenteric and the antimesenteric border (Figs. 20.2 and 20.3), in order to make the measurements reproducible both in the sane and in different hands. At the beginning of our experience with laparoscopic BPD, when the conversions to open surgery were more frequent, a check of intestinal lengths measured after conversion never showed a difference greater than 10 % between the two measurements [23], so, at least in our experience, we can refer to the intestinal lengths independently of the conditions (open or laparoscopic) in which the measurements were done. The length of 250 cm for the AL is the results of decades of experience, and should not be changed, unless a longer AL is requested to reduce to the minimum the weight loss, as in the above mentioned particular indications. An AL shorter than 250 cm entails a progressively increasing risk of PEM.
The CL length of 50 cm has been our gold standard since the completion of the early experimental phase, and it has remained the same during more than 30 years, always giving excellent and indefinitely maintained weight results. However, it demonstrated to be incompatible with a gastric volume smaller than 300 mL, the so-called very little stomach BPD causing, besides a 90 % loss of the excess weight, an incidence of 30 % PEM, with more than 10 % recurrent form requiring surgical revision [2, 18, 24]. This was confirmed by Sugerman et al. [14] who had overwhelming PEM problems when, leaving the very small gastric pouch, they converted standard RYGB into BPD with 200 cm AL and 50 cm CL (so-called distal gastric bypass). In our experience with BPD evolution, the progressive enlargement of the gastric remnant brought the PEM down to around 3 % with 1 % recurrence rate, and the additional lengthening of the AL from 200 to 250 cm made this important nutritional complication to essentially disappear, still leaving the permanent loss of the excess weight around 70 % [24]. With similar more than 300 mL gastric remnant, Clare [25] reported good results with 50 cm CL and equal AL and BPL (as our “half-half” BPD [26]), with little less weight loss than the standard model and only 2 % of hypoproteinemia. The same good results were obtained by Marceau [27] with the 50 cm CL standard BPD. Still, the problems remained of side effects, mainly represented by diarrhea, present in 13 % of operated patients.
More recently, Garcia et al. [28] compared standard BPD (50 CL – 200 AL cm) with a modified version (75–225 cm), showing an astonishing fall of malnutrition from 16 to 2 %, with slight lower weight loss. These findings go into the same direction of the preliminary ones of a prospective randomized trial we are carrying out on our patients, where a 75 cm CL results in modest reduction of weight loss accompanied by a significant reduction of side effects, mainly diarrhea but also gas problems and foul smelling stools.
For the rest, different intestinal lengths were used in modified RYGB in order to achieve a greater weight loss, especially in the super-obese patients. To understand what the authors actually did is made difficult due to the apparently different semantics they use when defining the intestinal limbs. First, Brolin et al. in 1992 proposed the “long-limb” RYGB [15], consisting of a “defunctionalized jejunum” (and then a BPL) of 150 cm compared with a BPL of 75 cm. The first operation caused a greater weight loss when compared to the second one only in the super-obese patients, when weight loss did not change in the morbidly obese ones. These results were confirmed by MacLean et al. in 2001 [16] and subsequently by Brolin himself in 2005 [29]. The confusion arises when the “very very long limb” RYGB is considered. This operation, described first by Murr et al. in 1999 [17] and then by Nelson et al. in 2006 [30], both belonging to the same group of the Mayo Clinic, Rochester, Minnesota, apparently consists of a gastric bypass with a short (60 cm) “pancreatobiliary limb,” which evidently corresponds to what we call BPL, a 100-cm “common channel,” and thus our CL, while all the rest of the small bowel, from the GEA to the EEA, ranging in length between 400 and 500 cm, which is evidently our AL, is named “Roux-limb.” The authors refer to the Sugerman “distal gastric bypass” [14], who uses the same terminology, that is, “Roux-limb” = AL. Therefore, the Brolin and MacLean “long-limb” is apparently named “Roux-limb” and corresponds to the biliopancreatic limb (BPL), while the definition “Roux-limb” in the Sugerman distal gastric bypass and in the Rochester group “very very long limb” is evidently referred to the alimentary limb (AL). This gross semantic confusion, that I have repeatedly indicated as one of the greatest problems in bariatric surgery (I even wrote a chapter in the Deitel’s book entitled “Semantics in obesity surgery” [31]), certainly does not help the reader to understand how these operations are structured, and, even less, how they work.
At the beginning of the introduction, when describing the BPD as a basic mechanism of action, we underlined that the mechanism is effective in causing a reduction, or, to say better, a limitation of calorie absorption only if the time of contact between food and biliopancreatic secretions is reduced enough to cause incomplete digestion and absorption of calorie rich aliment. At this point, in order to fully understand the importance of the intestinal limb lengths, it is important to remember that not necessarily the BPD operation causes weight loss. BPD simply leads the body weight to the value corresponding to the amount of daily calorie absorption which is allowed by its mechanism of action. As we said, there is for each BPD individual a maximum energy absorption capacity which, in that specific subject, if all the other conditions mentioned above remain constant, also remains constant and corresponds to a determined weight of stabilization. If the starting body weight of the BPD subject corresponds to a daily energy consumption higher than the operation energy absorption threshold, a negative calorie balance takes place causing the reduction of body weight which will stop only when the body energy expenditure will equal the energy absorption threshold. Assuming, for example, that the threshold is 1,600 kcal/day, corresponding to the body energy expenditure of 85 kg, the latter will be the weight of stabilization of that subject for that operation. Any starting body weight higher than this will be forced by the negative calorie balance to reduce to 85 kg, when the energy balance is reached between daily intestinal energy absorption and daily body energy expenditure. The result will be a weight loss equal to the starting body weight minus 85 kg. However, if the starting body weight is already stable at 85 kg, this means that the BPD individual is eating and consuming as much as the operation allows to absorb. Consequently, the operation causes no negative energy balance, neither weight change. The same obviously applies to the case of starting body weight lower than 85 kg. The BPD subject eats and consumes less energy than what the BPD allows to absorb, then again nothing would change in calorie balance and no weight change would occur.
BPD possesses some metabolic specific actions which are totally independent of the weight loss, the best known being the serum cholesterol lowering and the beneficial effect on glycemic control. Consequently, the morbidly obese patient, in case of hypercholesterolemia or type 2 diabetes mellitus, besides weight loss will also benefit of these two weight-independent actions. They depend on the structure of the operation, the first being due to the loss of bile salts in the colon, caused by the short segment of bile salt absorbing terminal ileum in continuity, which causes increased bile acid neosynthesis in the liver which happens at the expenses of the cholesterol pool. The beneficial action on type 2 diabetes, first reported by us in 1984 [26], is caused by hormonal changes due to the bypass of the duodenum and by the presence of aliments in the ileum. If these anatomic conditions are preserved, the two beneficial specific actions remain active also in case of no weight loss. Therefore, the operation is precious, because it can be used for the treatment of hypercholesterolemia and of type 2 diabetes mellitus also in lean individuals, with no risk of undue weight loss. In those cases, as we do not know which the exact value of energy absorption threshold will be, in order not to take the risk of undue weight loss, we deliberately increase its level by elongating by 50 cm both the CL and the AL, which become 100 and 300 cm, respectively, thus obtaining the preservation of the above specific actions with minimal or no weight loss at all [24, 32]. Obviously, in these conditions, an increase of energy intake would cause weight gain, but this only exceptionally happens, and the same applies to the hypothetical weight gain resulting from the better glycemic homeostasis.
In conclusion, the good knowledge and the wise use of the intestinal limbs absorption characteristics makes BPD an extremely ductile operation, as it can operate in such a wide range of action that it can cause from the greatest weight loss obtainable with any bariatric operation down to even no weight loss at all, still maintaining its specific actions, like the two mentioned above.
20.3 Clinical Management
20.3.1 Postoperative Management
One single antibiotic shot is given preoperatively, when thromboembolic prophylaxis is started, that is continued for the entire first postoperative month. The patient is ambulating the day of operation. Analgesia consists of morphine and ketorolac by means of a continuous delivery system for 3 days, with paracetamol on demand. Metoclopramide is administered routinely together with intravenous fluids, that is, 3 days.
NG tube is removed on the first postoperative morning. GEA transit is checked on the 2nd day by X-ray oral contrast (leak diagnosis is based on clinical signs). Clear liquids are allowed on the 3rd day, when i.v. fluids are discontinued. Solid food is given within the first week, according to the gastric emptying as shown by X-ray check. Patients are discharged on 4th or 5th day (the vast majority of them live far from Genoa).
Patients are called for routine follow-up visits (with all the laboratory exams done) at 1, 4, 12, 24, and 36 months. Subsequently, they are not called anymore, but strongly recommended to continue coming yearly, or at least sending their complete lab exams by fax or e-mail.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree