Human Papilloma Virus Infections: Introduction
|
Papillomaviruses (PVs) cause benign cutaneous and mucosal epithelial proliferations commonly called warts or verrucae. PV infections do not produce acute local or systemic signs or symptoms but induce slow, focal accumulations of keratinocytes. Lesions may remain subclinical for long periods or may enlarge into fulminating masses that persist for months or even years. Persistent lesions caused by certain types of human PV (HPV) can undergo neoplastic transformation.
(See Also Chapter 191)
PVs comprise a large family of small DNA viruses that infect humans and many other species.1 PVs are highly host-specific, meaning that those from one species do not induce papillomas in heterologous species, so HPVs infect only humans. Rabbit, bovine, and canine PVs have been used in animal models of viral infection because biologic experiments cannot be performed in humans using HPVs due to their oncogenic potential.
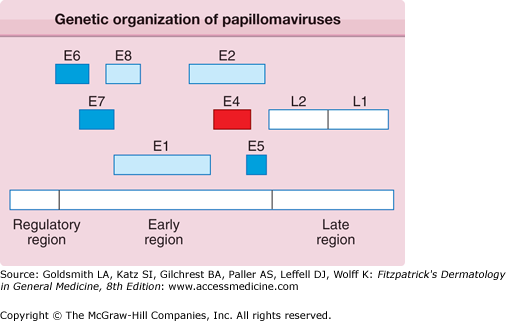
The PV genome is present within the viral particle as a single, covalently closed circle of double-stranded DNA. Each genome is composed of approximately 8,000 nucleotide base pairs and is approximately one-twentieth the size of a herpesvirus genome. Based on their DNA sequences, most human and animal PVs share a similar genetic organization (Fig. 196-1) that encodes only eight to nine related proteins.2 PV proteins are labeled as E (early) and L (late). 3 The E proteins are necessary for viral DNA replication and are not incorporated into the infectious virus particle. Because the E genes do not encode a DNA polymerase or thymidine kinase, PV replication is not selectively susceptible to inhibition by nucleoside analogues. Rather, PVs use host cell enzymes and factors to replicate their DNA genomes. The L1 and L2 genes encode the structural proteins that form the outer protein shell or capsid of the infectious viral particle, which is called the virion. The spherical virion measures approximately 55 nm in diameter and packages the viral DNA (Fig. 196-2).
Figure 196-1
All papillomaviruses genomes are composed of approximately 8,000 nucleotide base pairs, represented as a linear sequence but actually a closed circle of double-stranded DNA. The boxes depict the viral genes, each of which encodes a protein. The regulatory region does not encode proteins but is a DNA segment that controls expression of viral genes and replication of the viral genome. E6, E7, and E5 represent transforming genes; E1 and E2 coordinate replication and transcription of the viral genome; and the L1 and L2 proteins form the viral capsid. E4 encodes a protein that may be involved in the release of the virus from the cell’s keratin framework.
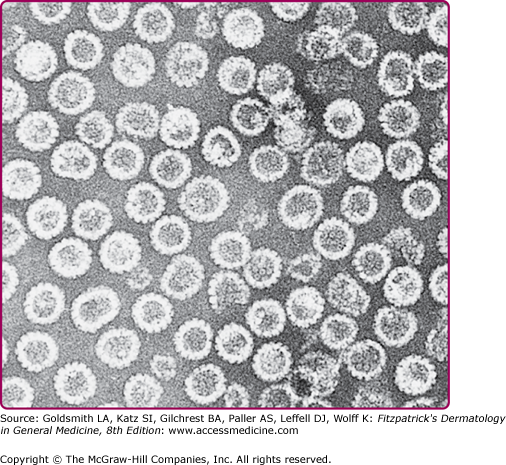
Figure 196-2
Transmission electron micrograph of human papillomavirus type 16 virus-like particles composed of the L1 and L2 proteins. These proteins were synthesized in cell culture and self-assembled into 55-nm particles that are morphologically similar to natural infectious virus except that they do not contain the viral DNA. The particles in the electron micrograph were purified from the cells. (Micrograph used with permission from Heather Greenstone.)
More than 100 HPV genotypes have been isolated and sequenced; the actual number of HPV genotypes is even higher.1,4 By convention, two HPV isolates are classified as the same type when a highly conserved sequence within their L1 genes are at least 90% identical.5 A new HPV genotype is recognized based on less than 90% homology to all other known HPVs within this DNA sequence.4 A subtype has 91%–98% identity, whereas a natural variant has less than 2% divergence to a known type in this region of L1.
Often associated with distinct regional predilection, histopathology, and biology, HPVs are often categorized as cutaneous (nongenital) and include genotypes such as HPV-1, -2, -3, and -4, whereas HPV-6, -11, -16, and -18 predominate in genital and mucosal infections. A large variety of HPV types such as HPV-5 and -8 have been isolated in epidermodysplasia verruciformis (EV), immunosuppressed individuals, and are also found to be present in normal skin (Table 196-1). PVs have also been classified by phylogenetic organization based on their DNA sequence relationships.6,7 The α genus includes a large set of cutaneous and genital-mucosal types and the β genus is primarily comprised of EV-types. Other genus classifications include more distantly related human and animal PV species. HPVs closely related by DNA sequence tend to induce similar lesions. Examples include highly related types such as 3 and 10, which induce flat warts; types 6 and 11, which cause genital–mucosal warts (condylomata acuminata); and types 5 and 8, which appear as scaly patches in EV.
HPV Type | Most Common Clinical Lesion | Less Frequent Lesion | Potential Oncogenicity |
|---|---|---|---|
1 | Deep plantar/palmar warts | Common warts | |
2, 4, 27, 29 | Common warts | Plantar, palmar, and mosaic warts | |
3, 10, 28, 49 | Flat warts | Flat warts in EV | HPV-10 rare in cervical and vulvar carcinomas |
7 | Butcher’s warts | ||
13, 32 | Oral focal epithelial hyperplasia | ||
5, 8, 9, 12, 14, 15, 17, 19–26, 36, 47, 50 | EV, warts in immunosuppression | Normal skin (?) | HPV-5, -8, -9 isolated from SCCs |
6, 11 | Anogenital warts, cervical condylomata | Bowenoid papulosis, oral warts; respiratory papillomatosis | Buschke-Löwenstein tumor; rare in penile, vulvar, cervical, and other urogenital tumors; “low risk” |
16, 18, 31, 33–35, 39, 40, 51–60 | Cervical condylomata; anogenital warts; bowenoid papulosis | Common warts | Genital and cervical dysplasias and carcinomas; rare in SCC of the digit; “high risk” |
Another important distinction is that specific HPV genotypes have malignant potential. This was first noted in EV. Warts that contain HPV types -5 and -8 undergo a high rate of malignant degeneration, whereas those induced by other HPV types, even in the same patient, tend to remain benign.8 Similarly, approximately 50% of cervical carcinomas contain HPV-16, with HPV-18, -45, -31, -33, -52 and -58 accounting for an additional 35%.9 Less frequent HPVs account for the remainder of cervical malignancies. This group is collectively referred to as high-risk types, whereas the low-risk types such as HPV-6 and -11 are isolated from benign cervical disease and genital warts, and identified in <1% of cervical malignancies.
HPV infection occurs through inoculation of virus into the viable epidermis through breaks in the epithelial barrier. Maceration of the skin is probably an important predisposing factor, as suggested by the increased incidence of plantar warts in swimmers who frequently use public pools. In rodent models of cervical HPV infection, mechanical disruption of the epithelium or inclusion of the mild detergent nonoxynol-9 dramatically increased the incidence of infection.10 Animal models using HPV virions demonstrate that attachment to heparan sulfate proteoglycans on the basement membrane is a required initial step in natural infection. A furin protease then cleaves L2, inducing a conformation change that allows binding to an unidentified basal cell receptor.11 This experimental model explains how PVs reserve infection for and specifically target epithelial basal cells. To establish persistent infection, the virus presumably must enter a stem cell or convert the infected cells to one with stem-like properties. After entry, a single copy or at most a few copies of the viral genome are maintained as an extrachromosomal plasmid or episome within the infected epithelial basal cell’s nucleus. When these cells divide, the viral genome also replicates and partitions to the progeny cell, which transports the viral infection into the differentiating layers of the epithelium. Viral RNA expression (transcription) is extremely low until the upper Malpighian layer, where viral DNA synthesis and genome amplification typically results in hundreds to thousands of copies per cell. The viral capsid proteins L1 and L2 (see Fig. 196-1) are synthesized in these upper epithelial strata and assemble into a tightly packed, very stable protein capsid. The newly synthesized viral DNA is packaged in the capsid, and mature virions accumulate in the nuclei of these upper level cells (see Fig. 196-2). The viral E1-E4 protein (the product of a spliced RNA from the E1 and E4 genes) may induce collapse of the cytoplasmic keratin filament network that surrounds the residual nucleus that contains assembled viruses.12 This is postulated to facilitate release of the virions from the cross-linked cytoskeleton of keratinocytes/corneocytes so that virus can be inoculated into another site or desquamated into the environment.
PV virions exfoliate along with the residual epidermal cells. PVs do not bud from the nuclear or plasma membrane as do many viruses such as herpes simplex or human immunodeficiency virus (HIV). Therefore, HPVs do not possess a lipoprotein envelope that leads to susceptibility to environmental conditions such as freezing, heating, or dehydration with alcohol that inactivate enveloped viruses. In contrast, PV virions are resistant to desiccation and to the detergent nonoxynol-9, although exposure of virions to formalin, strong detergents such as sodium dodecyl sulfate or prolonged high temperature reduces their infectivity.13,14 PVs can remain infectious for years when stored in glycerol at room temperature or in liquid nitrogen.
After experimental PV inoculation, a verruca usually appears within 2–9 months. This observation implies a relatively long period of subclinical infection that may act as an inapparent source of infectious virus. The rough surface of a wart can disrupt adjacent skin and enable inoculation of virus into adjacent sites, with the development of new warts over a period of weeks to months. Autoinoculation of virus into apposed skin is commonly seen on adjacent digits (see Fig. 196-3), mucosa (see Fig. 196-4), and in the anogenital region. Each new lesion results either from the initial exposure or spread from other warts. There is no convincing evidence for blood-borne dissemination.
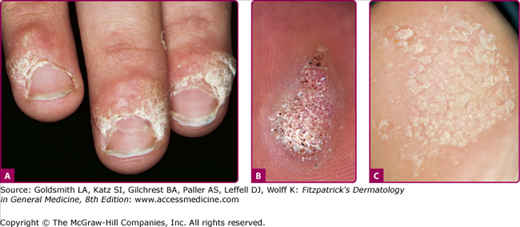
Figure 196-3
A. Common wart, periungual. Multiple, confluent, keratotic papules around the proximal periphery of the fingernails. B. Common wart, verruca plantaris with black dots of thrombosed capillaries. C. Common wart, mosaic plantar. A large hyperkeratotic plaque is seen on the heel, made up of multiple small coalescing warts.
The relative abundance of virus particles in a wart varies with the clinical setting and the HPV type. Newer lesions tend to contain more virions than do older verrucae. Plantar warts containing HPV-1 produce a high number of virions; anogenital verrucae typically have trace quantities of mature virus particles; common warts usually have intermediate numbers.
Because the viral episomes are at low copy and the L1 and L2 capsid proteins are not expressed in the lower epithelial levels in warts, the differentiation state of the infected epithelial cell influences viral transcription, signals initiation of viral DNA synthesis, and permits virion assembly. Further support for the belief that the production of virus particles depends on the state of epithelial differentiation is the fact that virion production decreases as benign papillomas progress toward dysplasia. Capsid proteins are never observed in HPV-infected malignant cells. The propagation of limited amounts of HPVs in cultured cells results from the ability to reproduce the physiologic milieu required for differentiation.15,16 Although it is possible to isolate infectious HPVs from cultured epithelial cells, the process is time-consuming, technically challenging, expensive, and limited to research laboratories. Therefore, laboratory culture for detection of HPV is not a practical diagnostic test.
Epidemiology
Acquisition of HPV depends on several factors, including the location of lesions, the quantity of infectious virus present, the degree and nature of the contact, and the general and HPV-specific immune status of the exposed individual. The roles of immunity and genetic susceptibility to PV infection are incompletely understood. The decrease in the frequency of warts with age implies that resistance to infection develops over time, and much of this resistance may be immunologic. In experimental PV infection in animals, resistance to virus challenge correlates with the presence of neutralizing anti-capsid antibodies. Serum or immunoglobulin G from resistant animals can provide protection through passive transfer.17,18 It is therefore likely that neutralizing antibodies account for at least some of the resistance to reinfection. Serum antibodies to viral capsids are detectable in some patients with current warts or a history of warts. These antibodies, as well as other host factors, may help to limit the spread of warts to new sites. Although humoral immunity may contribute to resistance to acquisition of infection, host cellular immune reactivity plays a critical role in wart regression. Individuals with impaired cell-mediated immunity are particularly susceptible to persistent HPV infection, and their infections are notoriously resistant to treatment. Warts are common in renal and solid organ transplant patients on immunosuppressive therapy, which may contribute to their increased risk of cutaneous malignancy.19,20 Although there are instances in which treatment of one or a few warts leads to resolution of many or all warts in nonimmunosuppressed patients, this outcome is the exception rather than the rule.
The reservoir of HPV is believed to be individuals with clinical and subclinical infection, as well as virus present in the environment. It is now apparent that HPV DNA is resident at very low levels on normal skin and in hair follicles. Using ultrasensitive polymerase chain reactions (PCR), a wide range of HPV, often of the β genus, is detected in the majority of individuals with both normal and suppressed host immunity.21–23 Whether this HPV DNA represents a subclinical state rather than true “latency” (i.e., nonreplicating and transcriptionally inactive virus) is unknown. Whether HPV DNA persists in a latent state in the epithelium after successful treatment of immunocompetent individuals also remains an unresolved issue. However, the fact that HPV DNA becomes undetectable in the majority of cervical infections, even by exquisitely sensitive PCR techniques, implies that complete viral clearance is possible.
Nongenital warts occur most frequently in children and young adults, in whom the incidence may exceed 10%.24,25 The age-specific incidence of nongenital warts differs from that of anogenital warts, which are uncommon in children. Anogenital warts behave as a sexually transmitted condition, and partners can transmit the virus with high efficiency.26 Penile lesions occur frequently in sexual contacts of women with cervical intraepithelial neoplasia.27,28
Although genital warts in children may be a consequence of sexual abuse, such warts in infants and children commonly result from virus inoculation at birth or from incidental spread from cutaneous warts (see Chapter 106). In contrast to anogenital lesions in adults, a significant proportion of genital warts in children contain HPV types that are usually isolated from nongenital warts.29–31
In addition to causing lesions on the external genitalia, genital-mucosal HPV types also infect the uterine cervix.32,33 This sexually transmitted infection, which may involve low- or high-risk HPV types, is especially common in sexually active women younger than 25 years of age. The majority of HPV DNA positive cervical infections are transient and remain histologically normal. Thus, even normal-appearing genital skin or mucosa may represent a reservoir for contagion. Studies of large populations throughout the world have documented the natural history of cervical infection and the association of high-risk HPV with cervical cancer. Persistence of HPV DNA strongly correlates with an increased risk for progression to cervical dysplasia and invasive cancer. High-risk HPV DNA has also been detected in clinically normal penile skin, although less is known of the epidemiology of genital HPV infection in men.34
The majority of respiratory (laryngeal) papillomas occur in infants and young children. The HPVs isolated from respiratory tract papillomas are often the same types as those of genital warts, especially HPV-6 and -11,35 and respiratory papillomatosis in young children is thought to result from seeding of the larynx and oropharynx during parturition.36 Although the epidemiologic correlation of condyloma in mothers of infants with respiratory papillomas has been documented,37 cervical and genital warts are common in the childbearing age women and respiratory papillomatosis of infants is rare. Because Cesarean section has attendant risk and is not fully protective, this is not routinely recommended for expectant mothers. Instead, treatment efforts should be aimed at reducing HPV infection before delivery. Oral and respiratory warts in adults are usually the consequence of oral–genital contact and is a significant risk factor for development of HPV-associated oropharyngeal cancers, most commonly with HPV-16.38
Approximately 50% of EV cases are inherited, usually with an autosomal recessive pattern. Studies to identify the HPV genotype in EV wart lesions are limited to research laboratories. In contrast to the finding of β-HPV types on normal skin, large numbers of these viruses are easily detected in EV lesions. Mutations in the EVER genes are reported to be present in a subset of EV individuals.39,40
Clinical Findings
The typical history is of a newly acquired, slowly expanding, persistent, and often scaly papule of the skin. Over several weeks to months, the appearance of additional nearby lesions indicates local spread and infers the diagnosis of HPV infection.
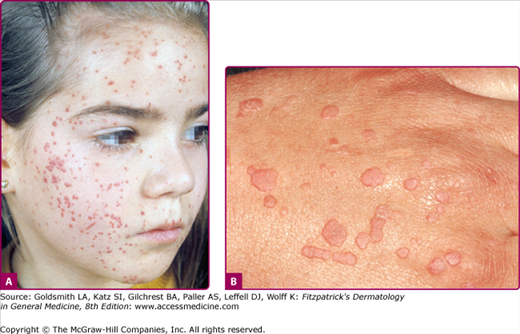
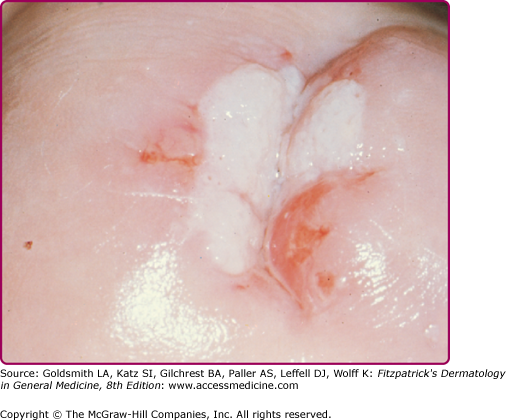
Warts are often classified by their anatomic location or morphology. As shown in Figs. 196-3, 196-4, 196-5, 196-6, 196-7, and 196-8, the physical appearance of warts varies. Common warts (verruca vulgaris) are scaly, rough, spiny papules or nodules (see Table 196-1). These may occur as single or grouped papules on the hands and fingers (see Figs. 196-3A and 196-5) or elsewhere (Fig. 196-7). Verrucae may be filiform and resemble cutaneous horns (Fig. 196-6). Flat warts (verruca plana) are 1–4 mm, slightly elevated, flat-topped papules that have minimal scale (see Fig. 196-8). These are most frequent on the face, hands, and lower legs. Plantar and palmar warts are thick, endophytic, and hyperkeratotic papules, which may be painful with pressure (see Figs. 196-3B and C). Punctate black dots (“seeds”) that represent thrombosed capillaries become evident after shaving off the outer keratinous surface. Mosaic warts result from coalescence of plantar or palmar warts into large plaques (see Fig. 196-3C). Some individuals with an apparently normal immunologic state develop exuberant warts of the palms or soles that are refractory to treatment. Butcher’s warts are verrucous papules, usually multiple, on the dorsal, palmar, or periungual hands and fingers of meat cutters, and may be infected with HPV-7.
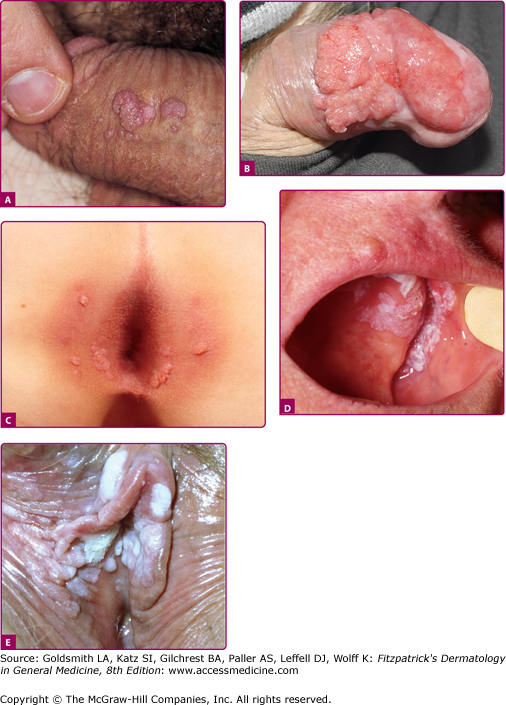
Anogenital warts (also known as condylomata acuminata, genital warts, or venereal warts) consist of epidermal and dermal papules or nodules on the perineum, genitalia, crural folds, and anus (196-9A,B,C and E). They vary in size and can form large, exophytic, cauliflower-like masses, especially in the moist environment of the perineum. Discrete 1- to 3-mm sessile warts may occur on the penile shaft. Lesions that resemble common warts also occur in this region but are unusual. Warts may extend internally into the vagina, urethra, and perirectal epithelium.
Figure 196-9
Mucosal warts. A. Multiple condylomata acuminata on the shaft of the penis B. Erythroplasia of the glans with exophytic SCC extending onto prepuce. C. Multiple perianal condylomata in a child. Sexual abuse must be considered. D: Oral florid papillomatosis with multiple, large verrucae. E. Multiple confluent condylomata on the labia minora, majora, and fourchette.
Bowenoid papulosis and erythroplasia are clinicopathologic entities in which high-risk HPVs have been identified.41 In bowenoid papulosis, papules, often multiple, appear as 2–3-mm lesions on the external male and female genitalia (see Chapters 77 and 78). Erythroplasia is characterized by a velvety, erythematous mucosal plaque with distinct borders (Fig. 196-9B). Histologically, there is cellular atypia resembling Bowen disease or squamous cell carcinoma (SCC) in situ. These lesions are usually infected with HPV-16, which suggests that bowenoid papulosis and erythroplasia may represent a precursor of penile and vulvar cancer. Erythroplasia can progress to invasive SCC (Fig. 196-9B). However, in bowenoid papulosis the rate of transition to frank malignancy is much lower for the external genitalia than for the cervix. These small papules should be treated also because they may represent a reservoir for the transmission of potentially oncogenic HPVs.
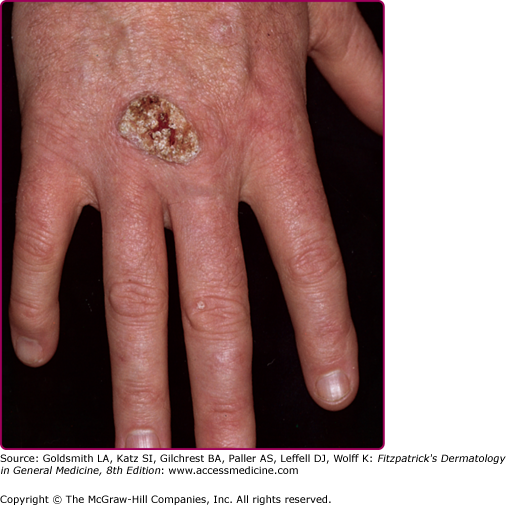
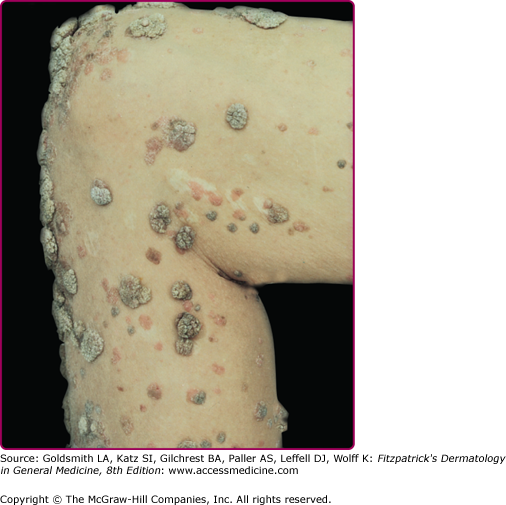
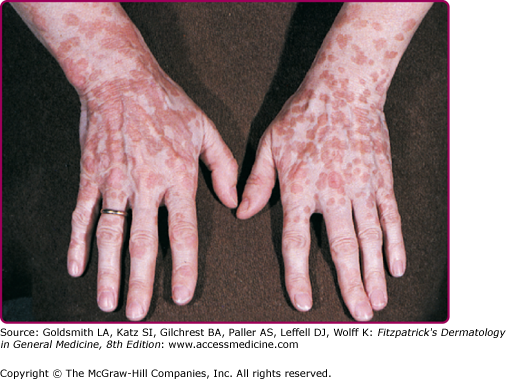
EV usually manifests in childhood with persistent and often widespread warts that do not regress due to unique susceptibility to specific HPV types. Individual lesions typically have either the appearance of flat warts (Fig. 196-10) or flat scaly red-brown macules that resemble pityriasis versicolor, particularly if the occur on the trunk. The presence of warts on large areas of the body is suggestive but not necessary to consider this diagnosis. Involvement of the cervix and oropharynx is rare. Failure to clear lesions despite adequate treatment is another indication of potential EV. Because warts in EV almost always recur after treatment, this implies a failure to mount an effective immune response to the HPV infection. Individuals with EV do not usually have frequent bacterial or other viral infections. Immunocompromised individuals, such as those with HIV infection, may have multiple warts that contain EV and other β-HPV types and are difficult to eradicate, but this susceptibility is acquired.42,43 SCCs in EV and immunosuppression usually arise in pityriasis-like lesions on sun-exposed areas (Fig. 196-11). Regional and distant metastases may occur. Although pityriasis-like lesions caused by any EV type are at risk of becoming malignant, this is higher for those caused by HPV-5 and -8.
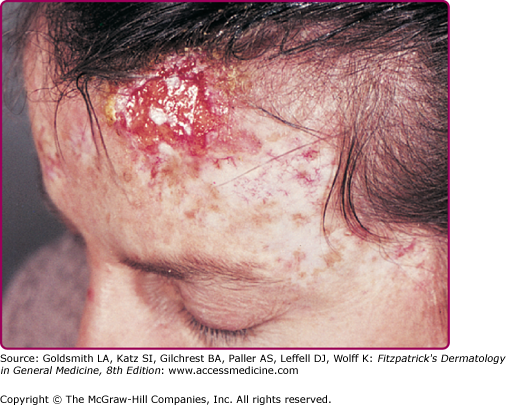
Figure 196-11
Invasive cancer in an epidermodysplasia verruciformis patient infected with numerous human papillomavirus (HPV) types, including HPV-5, -8, -9, -14, and others. In the tumor cells, HPV-5 DNA was detected in a high copy number. This large squamous cell carcinoma did not metastasize and did not recur after surgery. There are numerous actinic keratoses on the forehead.
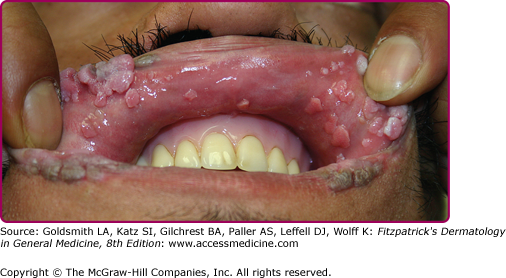
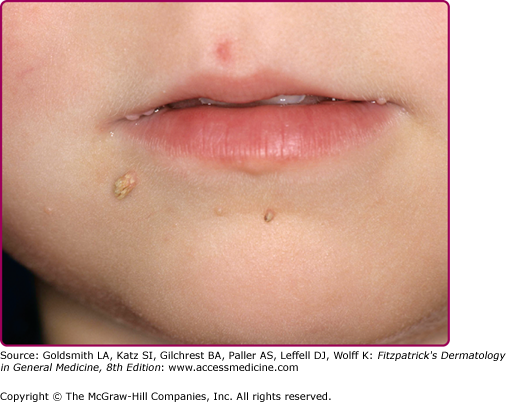
Oral warts are usually small, slightly elevated, soft, often pink or white papules that may be found on the buccal, gingival, labial mucosa (Fig. 196-4), tongue or hard palate. Verrucous, horny papillomas may occur on the palate. Mucosal lesions of the oropharynx, termed focal epithelial hyperplasia, also contain HPVs. In oral florid papillomatosis, multiple large verrucae appear within the oral cavity (see Fig. 196-9D). Progression to verrucous carcinoma may occur. Oral warts may result from genital contact. Warts may also occur in the urethra, usually when meatal warts are present. They may spread to the urinary bladder.
In respiratory (laryngeal) papillomatosis, multiple warts usually involve the larynx and may extend to the oropharynx and bronchopulmonary epithelia. Presenting symptoms commonly include hoarseness and stridor. Cases typically occur in infants or young children in whom the lesions may block the airway, but the condition may develop at any age. These HPV-associated papillomas may spontaneously remit, especially at puberty, but recurrences are frequent.
Cervical lesions are usually flat or slightly elevated and visualization may require colposcopy and enhancement after acetic acid application, which makes them appear as white patches (Fig. 196-12).
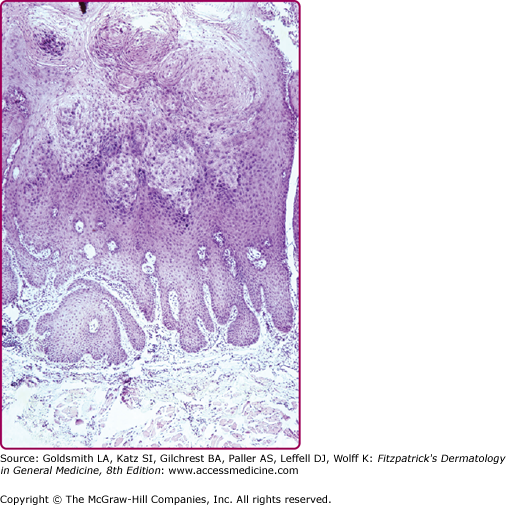
Verrucae consist of an acanthotic epidermis with papillomatosis, hyperkeratosis, and parakeratosis (Fig. 196-13). The elongated rete ridges often point toward the center of a wart. The dermal capillary vessels are prominent and may be thrombosed. Mononuclear cells may be present. Large keratinocytes with an eccentric, pyknotic nucleus surrounded by a perinuclear halo (koilocytotic cells or koilocytes) are characteristic of HPV-associated papillomas. Koilocytes visualized in a Papanicolaou (Pap) smear represent a hallmark of HPV infection. PV-infected cells may have small, eosinophilic granules and dense clumps of basophilic keratohyaline granules. These granules may be composed of or associated with the PV E4 (E1-E4) protein and are not conglomerates of virus particles. Flat warts have less acanthosis and hyperkeratosis and do not display parakeratosis or papillomatosis. Koilocytotic cells are usually abundant, indicating the viral origin of the lesion. Anogenital warts may have slight or extensive acanthosis and parakeratosis: they lack a granular layer as they are within or adjacent to a mucosal surface. The rete ridges often form thick bands extending extensively into the underlying, highly vascular dermis.
Flat warts have a similar histologic appearance whether in the general population or in patients with EV (Fig. 196-14). The granular and upper spinous layers contain many cells with perinuclear vacuolization. In EV, lesions containing HPV-5 or -8 have a characteristic basket weave hyperkeratosis with many large clear cells in the granular and spinous layers (Fig. 196-15).