Introduction
Hidradenitis suppurativa (HS) has a convoluted history with conflicting bodies of knowledge and a complicated pathogenesis. Since HS was first described in the 19th century, it has taken on various names and treatment strategies, leading to further confusion among researchers, physicians, and affected individuals. Alfred Velpeau and Aristide Verneuil are prominent historical figures who are credited with the discovery and characterization of HS centuries ago. Since then, many prominent physicians and researchers have contributed to our knowledge of this disease. Despite the longstanding history of investigation on HS, 150 years passed before development of the first staging system and there still remains no consensus on HS pathophysiology. As our understanding of HS grows, targeted therapies continue to evolve and help to clarify aspects of the pathogenesis as well as allow for optimal treatment of HS patients. Improved knowledge of the pathomechanisms in HS has slowly helped to reduce the controversy and mysticism surrounding this disease.
Historic Figures in Hidradenitis Suppurativa
The chronicle of HS began in 19th century France, where it was first described by Velpeau and Verneuil. Karl Marx, a famous 19th century German philosopher, is now also thought to have had HS, with earliest records of his disease dating just a few years after Verneuil’s first publications.
Alfred-Armand-Louis-Marie Velpeau (1795–1867)
Alfred-Armand-Louis-Marie Velpeau ( Fig. 1.1 ) was born in the Touraine village of Bréches, France on May 18, 1795.

Born of humble beginnings, he was expected to follow in his father’s footsteps, who was a farrier and “veterinary-artist.” However, Velpeau developed a strong interest in medicine, purchasing his first medical textbooks from money raised selling chestnuts while tending to his father’s cattle. After reading some “do-it-yourself”-type medical texts, he began providing opinions on his neighbors’ afflictions. Attempting to extinguish the sadness from a depressed young girl, he succeeded only in poisoning her with hellebore, which marked the turning point of his life.
When the local physician, Dr. Bodin, arrived to treat the girl, he became so impressed by Velpeau’s knowledge and determination that he introduced him to another member of the aristocracy, M. Duncan. Duncan shared in the fascination of Velpeau and invited him to join the tutoring lessons given to his children. After a year of demonstrating remarkable progress, Duncan introduced Velpeau to Vincent Gourand, surgeon at a hospital in Tours, and later to Piérre-Fidele Bretonneau, the head doctor of the hospital. Velpeau was only 21 years old (year 1816) at this point and was now an assistant to a renowned French physician of the time. Bretonneau treated Velpeau as a son over the next four years, training him in clinical medicine and pathology.
By 1820, at the age of 25, Velpeau accepted a position in the Saint Louis Hospital where he achieved awards in anatomy and physiology. In 1823, he was appointed “agrégé en médecine” (associate of medicine) with honors and in 1824 took appointments as junior surgical staff in various hospitals. After passing a higher degree in surgery, the “Chirurgical,” Velpeau was hired as surgeon of La Pitié in 1828. Five years later, at 38 years old, he was appointed as university chair of clinical surgery and went on to hold this position for the next 33 years.
During Velpeau’s tenure, he was said to have published works of over 340 titles and 10,000 pages. He authored texts on several topics, including surgical anatomy (1825), obstetrics (1830), operative medicine (1832), embryology (1833), as well as uterine and breast diseases (1854). Velpeau also gave his eponym to various anatomical structures, conditions, and innovations, notably Velpeau’s “canal,” “deformity,” “hernia,” and “pressure bandage.”
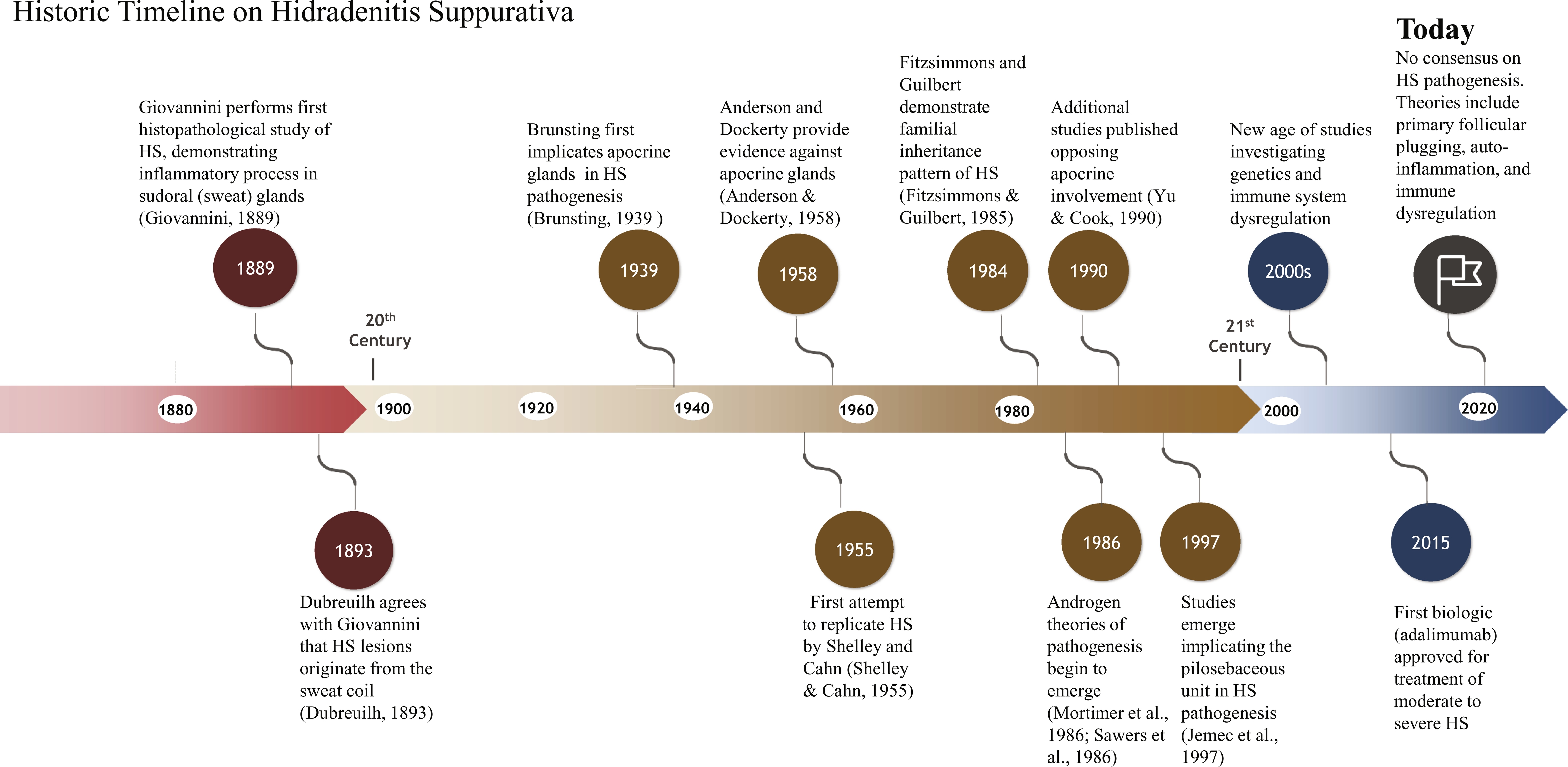
Furthermore, Velpeau is credited with being the first person to recognize and attempt to describe HS as a distinct disease process. In 1833, Velpeau described a “phlegmon tubériforme” (tuberiform phlegmnon) of the axilla, which he observed as inflammation of sebaceous follicles (frequently arising from rubbing irritation), which is occasionally painful and ends in suppuration. Velpeau also described an alternative but similar clinical aspect of HS, called “phlegmon érysipélateux” (erysipelas phlegmon), which included induration and tumors, and was always painful. Velpeau lamented the ignorance of authors in underestimating the dire consequences of this axillary inflammation.
Velpeau died on August 24, 1867, at the age of 72. He was a man of humble origins who ascended the ranks of the medical profession to become one of the prominent surgeons of his time. His contribution to dermatology is etched in history, as we credit him for his discovery of HS.
Aristide Auguste Stanislas Verneuil (1823–1895)
Aristide Auguste Stanislas Verneuil ( Fig. 1.2 ) was born in Paris, France on November 29, 1823.

In 1843 Verneuil was appointed Interne des Hôpitaux de Paris (Intern of Paris hospitals) and went on to graduate as a Doctor in Medicine in 1852. He became Professeur Agrégé (Associate Professor) at the Paris Faculty of Medicine in 1853 and appointed Surgeon of the Paris Hospitals in 1856, where he was the first official teacher of syphilis and other venereal diseases.
Verneuil held several successive appointments throughout his medical career, notably head of Lourcine Hospital, Le Midi Hospital (1865), Hôpital Lariboisière (1865), La Pitié (1872), and Hôtel-Dieu (1889), serving as Professor of Clinical Surgery (1872–1889) in La Pitié hospital, and Chair of Surgery at Hôtel-Dieu Hospital (1889–1892). Verneuil is also known for his positions as President of the Société de Chirurgie (1869), Charter member and later President of the Congress of Surgery, Member of the Académie de Médecine (1869), Member of the Académie des Sciences (1887), and Commander of the Legion of Honour.
Between 1854 and 1865, Verneuil published a number of articles on skin tumors and took particular interest in sudoral (sweat gland) tumors. This interest led him to Velpeau’s discovery. Although Velpeau had postulated that the origin of the abscesses found in HS patients was the sebaceous follicles, Verneuil hypothesized that they instead originated from the sweat glands. It is said that Verneuil had only personally observed a single case of acute inflammation of the sudoral glands, which was on a cadaver of a young girl. In his recorded notes, he observed inflammation of the sacral and gluteal region, multiple circumscribed and subcutaneous abscesses probably located in the sweat glands, and noted several pinhead-sized pustules filled with liquid but without any sign of inflammation. He reported unroofing the pustule to reveal a tiny red cavity in which a thin boar bristle or stylet could be introduced. The underlying channel was approximately one fifth to one third of a millimeter in depth, crossing the dermis and leading to a larger subdermal cavity filled with the same fluid, likely the early description of what is now known as a sinus tract.
Verneuil suspected that the suppuration was a result of necrosis of the sudoral glands with purulent excretion from the sudoral ducts, leading to accumulation of pus beneath the epidermis. Through clinical and histologic observation, Verneuil localized these abscesses to the sweat glands, with the caveat that he could not infer the etiology of the disease with so few observations. Verneuil died on June 11, 1895, leaving behind a rich legacy within the fields of surgery and dermatology. He was also the first to attempt describing the pathogenesis of HS.
Terminology
The 19th century’s identification of HS as a unique entity marked the start of debate regarding the proper naming convention for this disease. Sifting through the medical literature, one can find several different names to reference this one condition, including hidradenitis suppurativa, apocrine acne, apocrinitis, Velpeau’s disease, Verneuil’s disease, Fox-den disease, pyodermia sinifica fistulans, and acne inversa. ( Box 1.1 ) However, many of these names have since been abandoned or described as misnomers.
Velpeau’s disease
Verneuil’s diseas
Apocrinitis
Apocrine acne
Acne inversa
Hidradenitis suppurativa
In 1864, Verneuil named the disease “hidrosadénite phlegmoneuse,” the French term for “hidradenitis suppurativa.” This name was given based on Verneuil’s observation that the anatomical distribution of the disorder mirrored the characteristic distribution of sweat glands. The name was derived using Greek “hidros” meaning “sweat” and “aden” meaning “glands.” However, the proceeding indecision and controversy surrounding whether this condition was in fact its own separate disease led to the creation of various other names and eponyms.
In 1891, French physician Barthélémy regarded HS as part of a folliculitis and he introduced the new terms “acnitis” and “folliclis” to describe follicular and perifollicular inflammation of unknown origin. However, in 1893, French dermatologist Dr. Dubreuilh rejected the new terms in favor of “hidrosadenitis.”
At the beginning of the 20th century, nosological discussions arose regarding the connection between HS and acne conglobata, at which point French physician Dr. Spitzer proposed the name “dermatitis folliculitis et perifolliculitis conglobata” (dermatitis folliculitis and perifolliculitis conglobate). In 1949, another French physician, Dr. Degos, insisted that a distinction between acne conglobata and HS was nearly impossible to establish. Recognizing the difference between English and French views and noting that English literature commonly used the designation of “Verneuil’s disease,” French author Mouly encouraged others to acknowledge the work of Verneuil and named the condition “maladie de Verneuil” (Verneuil disease) in 1959.
In 1956, American dermatologists Pillsbury, Shelley, and Kligman named the coexistence of acne conglobata, hidradenitis suppurativa, and dissecting cellulitis of the scalp, the “follicular occlusion triad.” This decision was based on the common features of follicular hyperkeratinization, retention of keratin products, and secondary bacterial infection within all three conditions. Almost 20 years later, they proposed adding pilonidal sinus disease and updating the term to “acne tetrad” (also known as “follicular occlusion tetrad”).
More recently, the moniker “acne inversa” has gained favor based on histologic evaluations of the disease. In 1989, Plewig and Steger, two German dermatologists, suggested the name upon observing that the four components of the acne tetrad affected anatomical areas that were the inverse of the localizations of acne vulgaris. In 2005, American dermatologists Sellheyer and Krahl demonstrated histopathological evidence of the occlusion of hair follicles as well as observations of clinical and therapeutic similarities to acne. As a result, the authors insisted that “hidradenitis suppurativa” should be abandoned as a misnomer and replaced with “acne inversa.” However, opponents to the name “acne inversa” argue that histology alone does not define dermatology and that more research into the pathogenesis should be conducted. Thus, despite centuries of investigation, a general consensus regarding terminology for this disease had yet to be reached. “Hidradenitis suppurativa (HS)” is currently the most commonly used and recognized name for this disease.
Uncovering Hidradenitis Suppurativa Pathogenesis Studies Throughout History
Apocrine Gland Theory of Pathogenesis
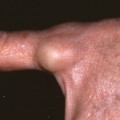
A 1889 publication by Italian physician Dr. Giovannini was likely the first histopathological study to support Verneuil’s hypothesis of the origin of HS by demonstrating the existence of an inflammatory process around the sweat glands associated with complete glandular destruction. In 1893, Dubreuilh agreed that HS lesions originated from the sweat coil. Apocrine glands were further implicated in HS pathogenesis in 1939 as Brunsting, an American dermatologist, studied the histology of several of his patients. Brunsting’s histologic observations led him to the theory that the apocrine structures and surrounding connective tissue were the initial locations of inflammation in HS. Furthermore, in his clinical description of the disease, he emphasized that lesions were located around the same anatomic regions that apocrine glands are situated.
HS was first attempted to be replicated in humans by American dermatologists Drs. Shelley and Cahn in 1955. Occlusion of the axillary skin of their subjects with belladonna tape for 2 weeks caused a quarter of them to develop subcutaneous nodules as seen in HS. Shelley and Cahn viewed histologic specimens of the subjects with clinical lesions, showing inflammation, keratinous plugging, and dilatation of the apocrine sweat duct without involvement of proximal hair follicles or sebaceous and eccrine glands. Shelley and Cahn concluded that hormonal dysregulation of the apocrine glands caused the hair follicle to become blocked that promoted inflammation with occasional secondary infection.
The work by Shelley and Cahn further supported the involvement of apocrine glands, and now, hormonal dysregulation in HS pathogenesis. This conclusion was due to histologic observations of known inflammatory changes within and around apocrine glands in HS patients. In addition, the increase in sex hormones during puberty, and young adulthood was thought to stimulate sex hormone receptors within apocrine glands to cause inflammatory changes in predisposed patients. Finally, HS and apocrine gland distribution in humans were similar: axillary, inguinal, perianal, perineal, mammary, inframammary, buttock, pubic, chest, scalp, retroauricular, and eyelid areas.
However, more recent studies suggest apocrine glands are not always involved in primary HS pathogenesis. American dermatologists Anderson and Dockerty were the first to provide evidence against apocrine glands being involved with primary pathogenesis of HS in 1958. They examined tissue samples from 64 individuals with HS and found that not all specimens had inflammation of the apocrine glands. Apocrine gland inflammation only occurred when there was also widespread inflammation involving eccrine and sebaceous glands, hair follicles, and blood vessels. Finally, there were no tissue samples with apocrine gland inflammation that spared the surrounding tissues. For these reasons, Anderson and Dockerty determined that apocrine glands were not essential for the primary pathogenesis of HS. In addition, two Welsh surgeons, Drs. Morgan and Hughes, found no difference in size or density of apocrine glands in HS subjects compared with control subjects.
English pathologists Drs. Yu and Cook conducted a study in 1990 that focused on examining the axillae of their HS patients. Most of the histologic specimens had atypical hair follicles, cysts, and dermal sinuses. The cysts had stratified squamous epithelium, laminated keratin, and most had hair follicles. A third of the subjects had apocrine gland inflammation when extensive inflammation was present throughout the subcutaneous tissue. This supported the findings of Anderson and Dockerty in that adnexal involvement did not always include apocrine glands. Findings by Attanoos et al. and Larralde et al. support Yu and Cook’s conclusion.
Pilosebaceous Follicle Theory of Pathogenesis
Danish dermatologist Dr. Jemec and pathologists Drs. Thomsen and Hansen studied sebaceous glands in the pathogenesis of HS in 1997. Some of their patient’s histologic specimens showed no involvement of sebaceous glands and minimal axillary, genital, inguinal, and facial seborrhea. They concluded that the pathogenesis of HS was not the same as acne, in which dysfunctional sebum production is typical.
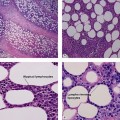
One of the most accepted theories of HS pathogenesis today is that primary follicular plugging causes inflammation and dilatation of the pilosebaceous unit. This causes inflammatory nodules and abscesses to form containing corneocytes, bacteria, sebum, and hair. Eventual rupture of these nodules and abscesses into the dermis propagates the spread of inflammation by invasion of neutrophils, lymphocytes, and histiocytes. Recurrent inflammation and secondary infection of the subcutaneous tissue creates sinus tracts and fistulas. Despite the evidence of follicular plugging being an important factor of HS pathogenesis, it is not a universally accepted theory and the cause of follicular plugging remains undetermined. The two most commonly proposed mechanisms are hyperparakeratosis or parakeratosis and anatomic abnormalities of the pilosebaceous unit.
To test these theories, Jemec and Gniadecka used ultrasonography to study hair follicles in subjects with HS. They found marked differences between control (from patients without HS) and HS follicles. In addition, structure and distribution of follicles were different for the axillary and genitofemoral regions. Interestingly, areas of skin that appeared to not be affected by HS still showed structural abnormalities of the follicle including distorted shapes and increased width variability. Genitofemoral areas had more variability to follicle width than axillary. Conversely, axillary areas had an overall increase in skin thickness and follicle distribution compared with genitofemoral areas. Despite their findings, Jemec and Gniadecka could not determine whether increased skin thickness and structural abnormalities of the hair follicles are primary factors of HS pathogenesis or secondary changes due to chronic inflammation.
Additional Theories of Past and Present
Today, additional theories of HS pathogenesis include genetic alterations and immune dysregulation. In the 1980s, English geneticists Drs. Fitzsimmons and Guilbert performed a series of studies that demonstrated the familial inheritance pattern of HS. Genome sequencing in Chinese families has since uncovered mutations in genes (NCSTN, PSENEN, PSEN1) that code for gamma-secretase, which cleaves the transmembrane receptor (Notch) involved in epidermal and follicular development. Other studies hypothesize that autoinflammation plays a role in disease pathogenesis, with notable elevations in several inflammatory cytokines (IL-1β, IL-10, IL-11, IL-17A, and CXCL9) observed in HS patients. A study by van der Zee et al. discovered that the cytokines IL-1β, and TNF-α, were elevated in HS lesions as well as in perilesional skin. The levels of these cytokines also showed trends toward a positive correlation with HS disease severity, making these markers suitable targets for medical therapy.
Hormonal influences have also been suspected to be a factor in HS pathogenesis by both clinicians and patients since the early 20th century. However, most of these reports are anecdotal with a limited number of studies supporting this theory. Beginning in 1986, two English studies found that antiandrogen therapy was an effective treatment for HS. Mortimer et al. found most subjects with HS had long-term improvement or complete remission of symptoms by reducing androgen levels. The same year, Sawers et al. found mixed results of antiandrogen therapy, in which some subjects had complete, long-term remission, while others experienced no benefit and excessive estrogenic side effects. In 2007, Canadian dermatologists Drs. Kraft and Searless reported results from a retrospective chart review, finding that the response to antihormonal therapy was superior to that of antibiotics. These findings support the theory that androgens could be a factor in HS pathogenesis. Despite all these discoveries, much remains unknown regarding the pathogenesis of HS and other mediators of this disease. The updated understanding of HS pathogenesis is discussed in 10, 11, 12 .
The Birth of Hidradenitis Suppurativa Staging Systems
The first staging system for HS was developed by Dr. Harry James Hurley Jr., an American dermatologist, in 1989. Hurley developed a staging system for HS by separating patients into three groups based on the extent of scar tissue and sinuses. Since its inception, the Hurley staging has been the most used system to assess HS severity. Hurley’s staging system was first introduced in a textbook chapter, not as a journal article.
The simplicity of the Hurley system has made its use convenient for clinicians. In 2003, Sartorius et al. suggested that the Hurley system is too basic to accurately evaluate treatment effects in clinical studies. Furthermore, the Hurley staging is not dynamic; a patient’s stage cannot be lowered if clinical signs improve. They proposed additions to the current Hurley staging system by including anatomic regions, number and types of lesions, distance between lesions, and the presence of normal skin in between lesions. Points are accumulated in each of these categories and added to give both a regional and total score. In addition, Sartorius suggested adding a visual scale for pain or using the dermatology life quality index (DLQI) when evaluating patients with HS. Hidradenitis Suppurativa Physician’s Global Assessment (HS-PGA) and Hidradenitis Suppurativa Clinical Response (HiSCR) are newer tools used to assess HS clinical severity and response to treatment. HS evaluation tools are discussed in greater detail in Chapter 13 .
A Historical Note on Hidradenitis Suppurativa Treatment
Currently, there are no single global standardized management guidelines for HS (see Chapter 14 ). There have been very few Level I evidence-based studies (randomized controlled trials or meta-analyses) for medical or surgical HS treatments, which adds to the difficulty in determining the most efficacious strategies. However, therapeutic approaches have been developed in response to evolving understanding of HS pathogenesis. Throughout history, various treatment modalities have been attempted to stop the progression of this disease, including medical therapies, surgical and in-office procedures, and lifestyle modifications. One can find a variety of therapies suggested for the treatment of HS in the literature, such as topical applications (e.g., hydrocortisone or clindamycin ), dry or wet warm compresses, ultraviolet light treatments, x-ray therapy, incision and drainage, antibiotics, exteriorization and curettage, electrocoagulation, and excision.
Lessons From the Past
The history of HS, though complicated and convoluted, can offer a wealth of information. Hidradenitis suppurativa, first named over 150 years ago, still remains a clinical conundrum today. The etiology of the disease has evolved from early understandings of primary apocrine gland inflammation to theories of primary follicular plugging, autoinflammation, and immune dysregulation ( Fig. 1.3 ). However, our lack of consensus on the underlying pathogenesis, as well as a standard naming convention, serves as a reminder of the many unknowns still surrounding this disease. As we move into an era of more targeted therapies for HS, it is important to review past and present studies to guide strategies for drug development. Research looking into the genetic profile of HS has provided additional insight into HS pathogenesis and inheritance patterns. Identifying causative mutations could allow for future studies on gene therapy and development of drugs targeted toward gamma-secretase. Similarly, further research into the autoinflammatory state and cytokine profile of HS has provided important implications for the management of HS with immunosuppressants, biologics, and small molecule inhibitors. Antiandrogen therapy has also shown efficacy in reducing HS symptoms and can be further explored. Diagnostic advances, along with surgical and procedural advances, will also allow for expeditious diagnosis and early treatment of HS to avoid devastating long-term sequelae.