Metastatic bone disease (MBD) afflicts more than half of the 1.2 million patients newly diagnosed with cancer annually.4,8
 Bony involvement can be a major source of morbidity and mortality if not treated appropriately.
Bony involvement can be a major source of morbidity and mortality if not treated appropriately.
 The femur is the long bone most commonly affected, with 25% of cases involving the proximal third of the femur.4,16,17
The femur is the long bone most commonly affected, with 25% of cases involving the proximal third of the femur.4,16,17
 The pelvis is the third most common site for metastasis.7
The pelvis is the third most common site for metastasis.7
 Seventy-five percent of all surgery for cancer that has metastasized to bone is performed in the hip area.17
Seventy-five percent of all surgery for cancer that has metastasized to bone is performed in the hip area.17
ANATOMY
 Metastatic foci to any part of the areas around the hip substantially compromise the mechanical integrity of the bone, placing the patient at high risk for fracture and subsequent nonunion.
Metastatic foci to any part of the areas around the hip substantially compromise the mechanical integrity of the bone, placing the patient at high risk for fracture and subsequent nonunion.
 The bony structure of the acetabulum consists of the anterior and posterior columns and their respective walls, which jut over laterally to cover the femoral head.
The bony structure of the acetabulum consists of the anterior and posterior columns and their respective walls, which jut over laterally to cover the femoral head.
 The anterior column is defined as the bone that extends from the iliac crest to the pubic symphysis.
The anterior column is defined as the bone that extends from the iliac crest to the pubic symphysis.
 The posterior column starts from the articulation of the superior gluteal notch with the sacrum and extends through the acetabulum and ischium to the inferior pubic ramus.
The posterior column starts from the articulation of the superior gluteal notch with the sacrum and extends through the acetabulum and ischium to the inferior pubic ramus.
 The acetabular dome, the superior weight-bearing region, consists of both the anterior and posterior columns and is contributed to by both walls.
The acetabular dome, the superior weight-bearing region, consists of both the anterior and posterior columns and is contributed to by both walls.
 The femoral head is not truly spherical; it is congruent only along the weight-bearing portion.
The femoral head is not truly spherical; it is congruent only along the weight-bearing portion.
 The principal and secondary bony trabeculations of the head, neck, and intertrochanteric area enable the head and neck arcade to withstand tremendous compressive and tensile forces.
The principal and secondary bony trabeculations of the head, neck, and intertrochanteric area enable the head and neck arcade to withstand tremendous compressive and tensile forces.
PATHOGENESIS
 The mechanism by which metastases occur is accounted for in a modified “seed/soil” theorem. Fewer than 1 in 10,000 neoplastic cells that escape into the circulation from the primary site are able to set up a metastatic focus. Metastasis, a complex, multistep process in which the cell first must break free, is a function of degradative enzymes such as collagenases, hydrolases, cathepsin D, and proteases. Once the cell invades the vascular channel, it circulates through the body.
The mechanism by which metastases occur is accounted for in a modified “seed/soil” theorem. Fewer than 1 in 10,000 neoplastic cells that escape into the circulation from the primary site are able to set up a metastatic focus. Metastasis, a complex, multistep process in which the cell first must break free, is a function of degradative enzymes such as collagenases, hydrolases, cathepsin D, and proteases. Once the cell invades the vascular channel, it circulates through the body.
 It is theorized that the cell is protected by a fibrin platelet clot. Clinical trials with heparin have not shown a significant change in metastatic outcome, however. Local factors such as integrins are instrumental in attracting the circulating metastatic cell to a particular remote tissue site. Once within the new tissue, the metastatic cell releases mediators such as tumor angiogenesis factor, inducing neovascularization, which in turn facilitates growth of the metastatic focus.
It is theorized that the cell is protected by a fibrin platelet clot. Clinical trials with heparin have not shown a significant change in metastatic outcome, however. Local factors such as integrins are instrumental in attracting the circulating metastatic cell to a particular remote tissue site. Once within the new tissue, the metastatic cell releases mediators such as tumor angiogenesis factor, inducing neovascularization, which in turn facilitates growth of the metastatic focus.
 Patients with advanced metastatic disease often experience dysfunction of hematopoietic and calcium homeostasis. They may develop a normochromic, normocytic anemia with leukocytosis. The increased number of immature cells, produced in response to the anemia and noted on the peripheral blood smear, is termed a leukoerythroblastic reaction.
Patients with advanced metastatic disease often experience dysfunction of hematopoietic and calcium homeostasis. They may develop a normochromic, normocytic anemia with leukocytosis. The increased number of immature cells, produced in response to the anemia and noted on the peripheral blood smear, is termed a leukoerythroblastic reaction.
Hypercalcemia may be seen in up to 30% of patients with extensive metastases, most commonly in myeloma, breast cancer, and non–small cell lung cancer.
 Blastic metastases are often painless and are associated with a lower incidence of pathologic fracture because the bone is not as severely weakened. Not all tumors that metastasize from the prostate to bone are blastic in nature, however. The lytic variants are painful and can cause pathologic fractures.
Blastic metastases are often painless and are associated with a lower incidence of pathologic fracture because the bone is not as severely weakened. Not all tumors that metastasize from the prostate to bone are blastic in nature, however. The lytic variants are painful and can cause pathologic fractures.
 Most tumors that metastasize from the breast to bone are blastic, but some demonstrate mixtures of blastic and lytic areas in the same bone. By taking serial radiographs and noting the appearance of bone metastases, it is possible to follow the progress of treatment with systemic hormone therapy or chemotherapy agents plus local radiation therapy. A favorable response may show a gradual conversion from a lytic to a blastic appearance as the pain decreases.
Most tumors that metastasize from the breast to bone are blastic, but some demonstrate mixtures of blastic and lytic areas in the same bone. By taking serial radiographs and noting the appearance of bone metastases, it is possible to follow the progress of treatment with systemic hormone therapy or chemotherapy agents plus local radiation therapy. A favorable response may show a gradual conversion from a lytic to a blastic appearance as the pain decreases.
 Bone destruction in lytic lesions occurs as a result of the biologic response by native osteoclasts to the tumor. Neovascularization is common. Among the tumors that are characteristic for this hemorrhagic response are thyroid carcinomas, renal cell carcinomas, and multiple myelomas.
Bone destruction in lytic lesions occurs as a result of the biologic response by native osteoclasts to the tumor. Neovascularization is common. Among the tumors that are characteristic for this hemorrhagic response are thyroid carcinomas, renal cell carcinomas, and multiple myelomas.
 Before surgical intervention is undertaken for these tumor types, it may be beneficial to perform a prophylactic embolization of the area to reduce perioperative bleeding. If a lesion is unexpectedly found to be aneurysmal at the time of surgical exploration, the friable tumor mass should be debulked rapidly down to normal bone, and the area should be packed until it can be stabilized with bone cement.
Before surgical intervention is undertaken for these tumor types, it may be beneficial to perform a prophylactic embolization of the area to reduce perioperative bleeding. If a lesion is unexpectedly found to be aneurysmal at the time of surgical exploration, the friable tumor mass should be debulked rapidly down to normal bone, and the area should be packed until it can be stabilized with bone cement.
NATURAL HISTORY
 Metastatic involvement of the musculoskeletal system is one of the most significant clinical issues facing orthopaedic oncologists. The number of patients with metastasis to the skeletal system from a carcinoma is 15 times greater than the number of patients with primary bone tumors of all types. About one-third of all diagnosed adenocarcinomas include skeletal metastases, resulting in about 300,000 cases per year. Furthermore, 70% of patients with advanced, terminal carcinoma demonstrate bone metastases at autopsy.
Metastatic involvement of the musculoskeletal system is one of the most significant clinical issues facing orthopaedic oncologists. The number of patients with metastasis to the skeletal system from a carcinoma is 15 times greater than the number of patients with primary bone tumors of all types. About one-third of all diagnosed adenocarcinomas include skeletal metastases, resulting in about 300,000 cases per year. Furthermore, 70% of patients with advanced, terminal carcinoma demonstrate bone metastases at autopsy.
 The carcinomas that commonly metastasize to bone are those of the prostate, breast, kidney, thyroid, and lung. One study showed that nearly 90% of patients with these types of carcinoma had bone metastases.
The carcinomas that commonly metastasize to bone are those of the prostate, breast, kidney, thyroid, and lung. One study showed that nearly 90% of patients with these types of carcinoma had bone metastases.
Among the carcinomas that less commonly metastasize to bone are cancers of the skin, oral cavity, esophagus, cervix, stomach, and colon.
 Because patients with MBD are surviving longer, surgeons must strive to perform an optimal reconstruction that can provide functional outcome for many years. However, once a pathologic fracture has occurred, a patient’s life expectancy is considerably shorter. Therefore, stringent surveillance by medical oncologists for bony metastases must be encouraged, with early referral to the orthopaedic surgeon before pathologic fractures occur.
Because patients with MBD are surviving longer, surgeons must strive to perform an optimal reconstruction that can provide functional outcome for many years. However, once a pathologic fracture has occurred, a patient’s life expectancy is considerably shorter. Therefore, stringent surveillance by medical oncologists for bony metastases must be encouraged, with early referral to the orthopaedic surgeon before pathologic fractures occur.
When patient survival is greater than 1 year after operative fixation for MBD, reoperation rates range from 3.1% to 42%.3
PATIENT HISTORY AND PHYSICAL FINDINGS
 In any patient with a history of cancer, especially those cancers that are well documented to metastasize to bone, any bone pain should raise suspicion for a metastatic focus.
In any patient with a history of cancer, especially those cancers that are well documented to metastasize to bone, any bone pain should raise suspicion for a metastatic focus.
 Pain at rest or at night that is or is not exacerbated with activity should heighten this suspicion.
Pain at rest or at night that is or is not exacerbated with activity should heighten this suspicion.
 The hip examination may or may not be abnormal.
The hip examination may or may not be abnormal.
IMAGING AND OTHER DIAGNOSTIC STUDIES
 A methodical approach is mandatory in the workup of a patient with presumed metastatic disease to bone to locate the primary tumor.
A methodical approach is mandatory in the workup of a patient with presumed metastatic disease to bone to locate the primary tumor.
A thorough history and physical examination must be completed before laboratory and radiographic analyses are done. The primary carcinoma may be detected on physical examination in as many as 8% of patients.
Laboratory analysis should include a complete blood count, erythrocyte sedimentation rate, renal and liver panels, alkaline phosphate, and serum protein electrophoresis.
 A plain chest radiograph and radiographs of known involved bones should follow.
A plain chest radiograph and radiographs of known involved bones should follow.
For metastases to the hip, an anteroposterior (AP) radiograph of the pelvis and full AP and lateral radiographs of the entire femur should be obtained.
About 45% of all primary tumors will be detected in the lung on the chest radiograph.
 The workup also should include a staging bone scan.
The workup also should include a staging bone scan.
If this scan is negative, myeloma should be suspected.
If the scan is positive, a lesion may be found at a more convenient biopsy site.
Bone scanning is more sensitive than plain radiographs in detecting early lesions.
 Computed tomography (CT) scans of the chest, abdomen, and pelvis should be performed.
Computed tomography (CT) scans of the chest, abdomen, and pelvis should be performed.
CT of the lung can detect up to 15% of primary tumors missed on the plain radiograph.
 The use of positron emission tomography scanning, either in isolation or in conjunction with CT, is becoming more common in the workup of patients with possible metastatic cancer.
The use of positron emission tomography scanning, either in isolation or in conjunction with CT, is becoming more common in the workup of patients with possible metastatic cancer.
 These studies, in combination with a well-planned biopsy, will reveal the primary cancer for most patients.
These studies, in combination with a well-planned biopsy, will reveal the primary cancer for most patients.
Routine radiographic screening studies in search of early metastatic disease are not very helpful. Lytic changes become evident on routine radiographs only when cortical destruction approaches 30% to 50%.
 If a lesion is detected about the hip in the anatomic areas as described earlier, and a detailed pelvic and hip CT scan has not been performed within the past 6 to 8 weeks, one should be ordered.
If a lesion is detected about the hip in the anatomic areas as described earlier, and a detailed pelvic and hip CT scan has not been performed within the past 6 to 8 weeks, one should be ordered.
Intravenous contrast medium is not necessary.
A recent CT scan is particularly important in the preoperative planning for an acetabular reconstruction.
DIFFERENTIAL DIAGNOSIS
 Prostate cancer
Prostate cancer
 Breast cancer
Breast cancer
 Kidney carcinoma
Kidney carcinoma
 Thyroid carcinoma
Thyroid carcinoma
 Lung carcinoma
Lung carcinoma
 Myeloma
Myeloma
 Lymphoma of bone, although less common, can mimic these diagnoses.
Lymphoma of bone, although less common, can mimic these diagnoses.
 For a patient older than the age of 40 years, with no known history of metastatic carcinoma to bone, the osteophilic malignancies mentioned earlier must be considered and evaluated as described.
For a patient older than the age of 40 years, with no known history of metastatic carcinoma to bone, the osteophilic malignancies mentioned earlier must be considered and evaluated as described.
NONOPERATIVE MANAGEMENT
 Nonsurgical management of metastatic carcinoma to bone includes observation, radiation treatment, and hormonal or cytotoxic chemotherapy.
Nonsurgical management of metastatic carcinoma to bone includes observation, radiation treatment, and hormonal or cytotoxic chemotherapy.
 Radiation is reserved for palliative intervention. Each patient’s suitability for radiation therapy must be carefully determined. The histologic type of disease, extent of disease, prognosis, marrow reserve, and overall constitution must be assessed.
Radiation is reserved for palliative intervention. Each patient’s suitability for radiation therapy must be carefully determined. The histologic type of disease, extent of disease, prognosis, marrow reserve, and overall constitution must be assessed.
 Impending lesions about the proximal femur and acetabulum should dissuade the orthopedist from nonoperative management, particularly in renal cell and thyroid carcinoma, where bony destruction is likely to progress despite the best nonsurgical modalities.
Impending lesions about the proximal femur and acetabulum should dissuade the orthopedist from nonoperative management, particularly in renal cell and thyroid carcinoma, where bony destruction is likely to progress despite the best nonsurgical modalities.
 For a patient who has sustained a pathologic fracture secondary to metastatic carcinoma, the average survival time is 19 months.
For a patient who has sustained a pathologic fracture secondary to metastatic carcinoma, the average survival time is 19 months.
Each histologic type has varying lengths of survival: prostate, 29 months; breast, 23 months; renal, 12 months; and lung, 4 months.
Moreover, each type of carcinoma exhibits varying radiosensitivity: prostate and lymphoreticular carcinomas, excellent; breast carcinoma, intermediate; and renal and gastrointestinal carcinomas, poor.
 When radiation therapy is used appropriately, 90% of patients gain at least minimal relief, with up to two-thirds obtaining complete relief. Seventy percent of patients who are ambulatory retain their ability to ambulate after radiation therapy to the lower extremities.
When radiation therapy is used appropriately, 90% of patients gain at least minimal relief, with up to two-thirds obtaining complete relief. Seventy percent of patients who are ambulatory retain their ability to ambulate after radiation therapy to the lower extremities.
Systemic radioisotopes also may be used. Strontium 89 mimics calcium distribution in the body and has shown promise in clinical applications.
 When a patient has sustained a true pathologic fracture (rather than an impending lesion), surgical stabilization usually is indicated, with subsequent radiation therapy.
When a patient has sustained a true pathologic fracture (rather than an impending lesion), surgical stabilization usually is indicated, with subsequent radiation therapy.
Because of poor bone quality, bone cement often must be used to augment the fixation.
 Hormonal therapy has an important role in the management of metastatic breast and prostate cancer. Fortunately, these agents are easy to administer and have few side effects.
Hormonal therapy has an important role in the management of metastatic breast and prostate cancer. Fortunately, these agents are easy to administer and have few side effects.
 For breast cancer, medical hormonal manipulation can be done by use of antiestrogens, progestins, luteinizing hormone–releasing hormone, or adrenal-suppressing agents.
For breast cancer, medical hormonal manipulation can be done by use of antiestrogens, progestins, luteinizing hormone–releasing hormone, or adrenal-suppressing agents.
Tamoxifen is effective in 30% of all breast cancer cases; its effectiveness increases to 50% to 75% of cases in which the tumor is known to be estrogen receptor– and progesterone receptor–positive.
Surgical ablation (oophorectomy) also may have a role in certain cases.
 In some cases of prostate cancer, reduction in testosterone levels via bilateral orchiectomy or administration of estrogens or antiandrogens may produce dramatic results.
In some cases of prostate cancer, reduction in testosterone levels via bilateral orchiectomy or administration of estrogens or antiandrogens may produce dramatic results.
Estrogens are no longer used as a first-line agent because of the risk of cardiovascular complications.
 Cytotoxic chemotherapy is used extensively in the treatment of adenocarcinoma. However, in older patients with advanced disease, the side effects of the drugs may be too severe.
Cytotoxic chemotherapy is used extensively in the treatment of adenocarcinoma. However, in older patients with advanced disease, the side effects of the drugs may be too severe.
SURGICAL MANAGEMENT
 For cases involving the periacetabular area, femoral head, neck, and intertrochanteric area, cemented femoral arthroplasty components are an important surgical option for impending and realized fracture management.
For cases involving the periacetabular area, femoral head, neck, and intertrochanteric area, cemented femoral arthroplasty components are an important surgical option for impending and realized fracture management.
 The goals for surgical intervention in the patient with metastatic carcinoma to bone are relief of pain; prevention of impending pathologic fracture; stabilization of true fractures; enhancement of mobility, function, and quality of life; and, for some, improved survival.
The goals for surgical intervention in the patient with metastatic carcinoma to bone are relief of pain; prevention of impending pathologic fracture; stabilization of true fractures; enhancement of mobility, function, and quality of life; and, for some, improved survival.
 It is generally agreed that a patient must have a life expectancy of at least 6 weeks to warrant operative intervention.
It is generally agreed that a patient must have a life expectancy of at least 6 weeks to warrant operative intervention.
 Cancer patients, regardless of their age, may have increased difficulty protecting their fixation device or prosthesis secondary to systemic debilitation. Accordingly, rigid fixation, with polymethylmethacrylate (PMMA) augmentation as needed, is mandatory.
Cancer patients, regardless of their age, may have increased difficulty protecting their fixation device or prosthesis secondary to systemic debilitation. Accordingly, rigid fixation, with polymethylmethacrylate (PMMA) augmentation as needed, is mandatory.
Preoperative Planning
 In many cases, the diagnosis of metastasis to the proximal femur will be made before a fracture occurs. In these cases, it is the responsibility of the orthopaedic surgeon to decide whether the patient should receive some form of internal stabilization before radiation therapy is begun. A CT scan of the involved area will help make this decision.
In many cases, the diagnosis of metastasis to the proximal femur will be made before a fracture occurs. In these cases, it is the responsibility of the orthopaedic surgeon to decide whether the patient should receive some form of internal stabilization before radiation therapy is begun. A CT scan of the involved area will help make this decision.
 Criteria for the performance of a prophylactic stabilization procedure include the following:
Criteria for the performance of a prophylactic stabilization procedure include the following:
Fifty percent cortical lysis
A femoral lesion greater than 2.5 cm in diameter
An avulsion fracture of the lesser trochanter
Persistent pain in the hip area 4 weeks following the completion of radiation therapy
 A Mirels score (Table 1) also may help in treatment decision making for hip and femoral lesions.
A Mirels score (Table 1) also may help in treatment decision making for hip and femoral lesions.
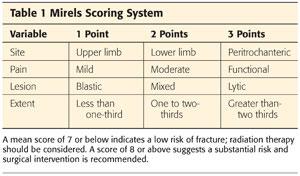
As elucidated in the Mirels score, the peritrochanteric area in general is at high risk for fracturing.
These criteria are not perfect, and large errors arise in estimation of the load-bearing capacity of the bone. For example, no system takes into account the histologic subtype, preexisting osteoporosis, and functional demands. Objective quantification of pain in the Mirels score is controversial as well.
Periacetabular Lesions and Impending and Realized Fractures
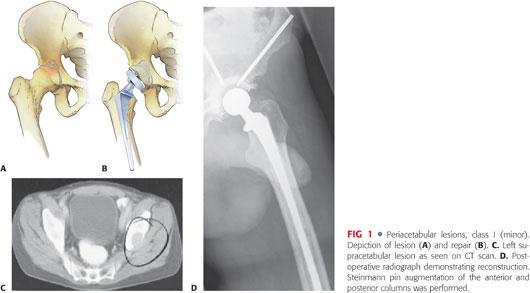
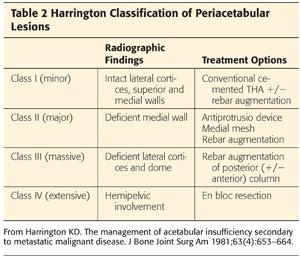
 Class I (minor): Lateral cortices, superior wall, and medial wall are intact (FIG 1). Treat with conventional cemented acetabular component with or without rebar (anchorage with large fragment screws) as needed (Table 2).
Class I (minor): Lateral cortices, superior wall, and medial wall are intact (FIG 1). Treat with conventional cemented acetabular component with or without rebar (anchorage with large fragment screws) as needed (Table 2).
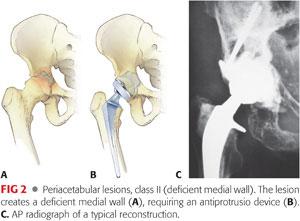
 Class II (major): Deficient medial wall (FIG 2) requires an antiprotrusio device, medial mesh, or rebar (Table 2).
Class II (major): Deficient medial wall (FIG 2) requires an antiprotrusio device, medial mesh, or rebar (Table 2).
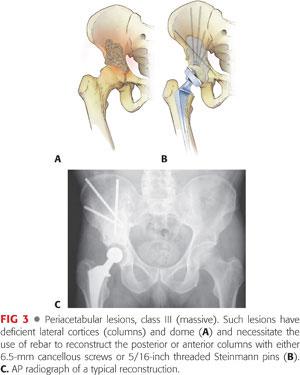
 Class III (massive): Deficient lateral cortices and dome (FIG 3) mandate rebar augmentation of the posterior and sometimes the anterior columns; 6.5-mm cancellous screws or 5/16-inch Steinman pins are recommended (Table 2).
Class III (massive): Deficient lateral cortices and dome (FIG 3) mandate rebar augmentation of the posterior and sometimes the anterior columns; 6.5-mm cancellous screws or 5/16-inch Steinman pins are recommended (Table 2).
 Class IV: Resection is mandatory for attempted cure. Such cases should be referred to an orthopaedic oncologist and are beyond the scope of this chapter.
Class IV: Resection is mandatory for attempted cure. Such cases should be referred to an orthopaedic oncologist and are beyond the scope of this chapter.
Femoral Head and Neck
 Impending fractures
Impending fractures
Femoral head involvement is a reason to perform arthroplasty (FIG 4).
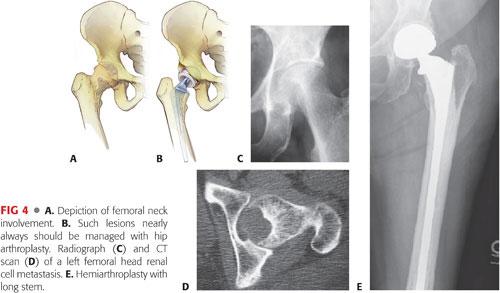
Modest femoral neck lesions may be stabilized with a reconstruction nail, with the exception of renal cell and thyroid carcinomas, in which cases arthroplasty is recommended.
 Realized fractures
Realized fractures
Rarely heal
Internal fixation device failure is common.
Procedure of choice: replacement arthroplasty
• The decision to perform bipolar versus total hip arthroplasty is a result of acetabular involvement, preexisting arthritis, and life expectancy.
• Acetabular disease may go unrecognized on plain radiographs in up to 83% of cases. Pelvic CT is imperative.
Long-stem prostheses may be used for extensive femoral involvement, but attention must be paid to cement deployment during the early cure stage, use of a long laparoscopic sucker, or venting.
Peritrochanteric Neck
 Impending fractures
Impending fractures
An intramedullary (IM) reconstruction-type device is strongly recommended. Screw and side plate constructs have a high failure rate (FIG 5A).
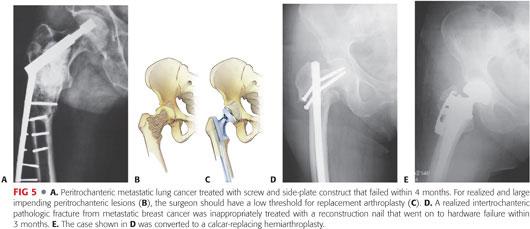
Long-stem stabilization and protection of the entire bone should be considered.1
For renal cell and thyroid cancer, the surgeon should proceed with cemented calcar-replacing arthroplasty.
 Realized fractures
Realized fractures
Cemented calcar-replacing arthroplasty is the only appropriate option (FIG 5B–E).
Subtrochanteric
 Impending fractures
Impending fractures
With the exception of renal cell and thyroid carcinoma, a cephalomedullary nail reconstruction is appropriate when bone loss is not extensive (FIG 6A–E).

Otherwise, proximal femoral replacement is necessary.
 Realized fractures
Realized fractures
Cemented proximal femoral replacement is the only viable option to restore the patient to ambulatory status (FIG 6F,G).
Long-stem cemented femoral arthroplasty use in patients with MBD remains controversial. Some surgeons are cautious in the general use of cemented long-stem femoral arthroplasties in patients with MBD because of the risk of cardiopulmonary insult and collapse.
Combining the use of bone cement with a long-stem femoral component further increases the possibility of complications, especially in a patient with MBD who has poor-quality bone and severe preexisting medical conditions. Deciding whether femoral stability from a cemented long-stem arthroplasty is worth the increased risk of a life-threatening cardiopulmonary embolic event is difficult. Certain steps listed in the following sections have been shown to minimize this risk, warranting long-stem use in cases of extensive femoral disease.14
Positioning
 Hip arthroplasty can be performed in either the supine or lateral decubitus position, but it is strongly recommended that the patient be placed in the decubitus position for anything other than a routine arthroplasty. This enables the surgeon to perform arthroplasty as well as extensive instrumentation of the posterior column when necessary.
Hip arthroplasty can be performed in either the supine or lateral decubitus position, but it is strongly recommended that the patient be placed in the decubitus position for anything other than a routine arthroplasty. This enables the surgeon to perform arthroplasty as well as extensive instrumentation of the posterior column when necessary.
 Reconstruction of impending proximal femoral lesions can be performed with the patient placed in the supine position on a fracture table that allows insertion of a cephalomedullary device and interlocking screws.
Reconstruction of impending proximal femoral lesions can be performed with the patient placed in the supine position on a fracture table that allows insertion of a cephalomedullary device and interlocking screws.
Approach
 Standard, but sometimes expanded, anterior, anterolateral, and posterior approaches may be used to access the acetabulum.
Standard, but sometimes expanded, anterior, anterolateral, and posterior approaches may be used to access the acetabulum.
 For posterior column instrumentation, an extensile posterior approach is recommended.
For posterior column instrumentation, an extensile posterior approach is recommended.
TECHNIQUES
 Periacetabular Reconstruction
Periacetabular Reconstruction
Rigid fixation of the acetabular component is critical to success. The preoperative CT and plain radiographs must be evaluated carefully before surgery (TECH FIG 1A,B).
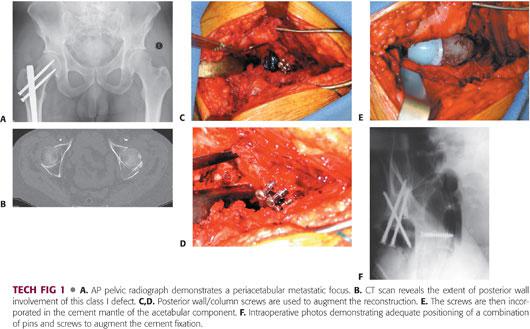
Class I defects can be managed with a conventional cemented acetabular component, with or without augmentation of fixation with large fragment screws (TECH FIG 1C–E).
Class II defects
An antiprotrusio cage or a similar device must be used.
Any flanges or screws must be attached to healthy bone.
A posterior approach without a trochanteric osteotomy is usually adequate.
Nonunion of a trochanteric osteotomy is a major concern in patients with cancer and should be avoided unless absolutely necessary.
However, visualization of the posterior column is critical to confirm its mechanical integrity; therefore, an incision of adequate size must be used.
Class III defects
An extensive posterolateral or lateral approach usually is chosen to deploy 6.5-mm cancellous screws or Steinmann pins under direct visualization with palpation of the sciatic notch and its contents.
Trochanteric osteotomy is operator dependent, but the surgeon must factor in the higher nonunion at this site given the patient’s underlying condition and possible adjuvant radiation therapy.
If the disease is locally advanced, an extensile iliofemoral approach may be necessary to visualize the inner as well as the outer pelvis.
The surgeon places his or her index finger into the sciatic notch and then aims the rebar screw or pin parallel to the notch into the posterior column of bone toward the sacral ala.
Because threaded pins do not give adequate proprioceptive feedback, the surgeon is encouraged to use a 3.2-mm drill bit with a subsequent depth gauge to confirm that the drill hole has adequate wall integrity.
At least two—preferably three or more—screws or pins are necessary to anchor the reconstruction. Intraoperative radiographs can be taken as needed (TECH FIG 1F).
Although anterior column fixation is less important than posterior column fixation, if the anterior column is compromised, Steinmann pins may be deployed antegrade from the anterior crest into the acetabular defect.
Some surgeons use targeting jigs, but we prefer to use a careful freehand technique with the nondominant hand in the defect to target the pin.
These anterior pins are cut flush with the crest after they are deployed to the appropriate depth in the defect, ideally capturing the ilium.
With the rebar in place and sunk to a depth that does not interfere with the acetabular component also being sunk to the correct depth, version, and verticality, mesh or similar material is placed to limit cement extrusion.
The acetabular component is then cemented into place, making sure to get the PMMA fully interdigitated with the rebar.
 Long-Stem Cemented Femoral Components
Long-Stem Cemented Femoral Components
We prefer to use long-stem femoral components during hip arthroplasty for MBD, with a minimum stem length of 300 mm (TECH FIG 2A).
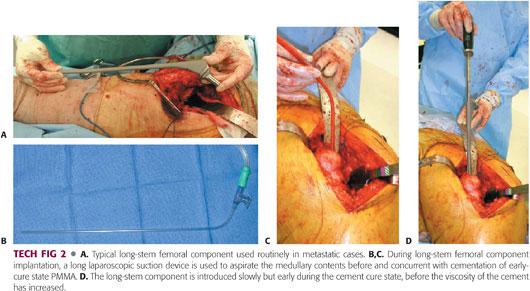
Various surgical techniques have been proposed to reduce perioperative canal debris or IM pressurization.
Low-viscosity cement, IM venting, retrograde injection, thorough IM lavage, and intraoperative canal suctioning during cementing may decrease embolic events and decrease perioperative complications.
Femoral preparation and component placement are performed in a similar systematic fashion.
After the femoral neck cut is completed with an oscillating saw, the canal is prepared with flexible reaming and broaching.
The canal is suctioned between subsequent reamers with a long laparoscopic suction device (Sigmoidoscopic Suction device, Ref # 0033050; ConMed Corp., Utica, NY; TECH FIG 2B,C). The canal is then thoroughly brush-lavaged using the Pulsavac (Zimmer, Warsaw, IN) system.
Three batches of Surgical Simplex P bone cement (Stryker, Mahwah, NJ) are mixed with 3.6 g of tobramycin for femoral cementation because of the patient’s immunocompromised condition. We prefer Simplex P bone cement because of its low viscous qualities on immediate mixing. Once the cement is mixed (<1 minute), it is immediately injected into the femur in its early, liquefied cure state using a long cement gun.
The long laparoscopic suction device (ConMed Corp.) is used to aspirate the canal immediately before and during insertion of the PMMA.
The femoral prosthesis is then slowly inserted into the femoral canal and allowed to settle with minimal manual force to avoid high-peak pressurization (TECH FIG 2D).
All excess cement is removed and the implant is held in position until the PMMA has hardened.
No distal venting is performed to avoid potential distal stress risers and minimize operative time. No cement restrictors are used.
 Calcar-Replacing Hip Arthroplasty
Calcar-Replacing Hip Arthroplasty
In the presence of peritrochanteric bone loss without subtrochanteric extension, a cemented calcar-replacing implant may be used.
The surgeon may still consider using a longer cemented stem if the appropriate precautionary steps, as outlined earlier, are taken.
 Proximal Femur Replacement
Proximal Femur Replacement
For proximal femoral replacement, a long posterolateral incision is made to expose the proximal fourth to third of the femur.
The iliotibial band is incised longitudinally to permit anterior and posterior exposure.
The gluteus maximus is carefully split, with concurrent meticulous ligation of perforating arterioles.
Time is taken to localize and protect the sciatic nerve in the retrogluteal area, where it lies immediately behind the external rotators.
The abductors are defined, and the greater trochanter is osteotomized and preserved if it is not extensively involved with tumor.
If the greater trochanter is too compromised, the abductors are transected at their tendinous attachment.
The vastus lateralis muscle is reflected anteriorly, ligating the perforators serially. The main blood supply enters anteriorly.
The external rotators are taken down using the surgeon’s preferred technique.
However, the hip capsule should be preserved as carefully as possible because it is instrumental in stabilizing the endoprosthetic reconstruction. It is recommended that the capsule be incised longitudinally, with the incision extending anteriorly over the neck, and detached circumferentially.
It is strongly recommended that the entire limb, including the foot, be prepped in sterile fashion so that a distal pulse examination may be performed intraoperatively.
The hip is dislocated anterolaterally.
The acetabulum is inspected and assessed for possible reconstruction.
Femoral resection level is determined by the lesion or fracture (TECH FIG 3A,B).
If the fracture under management is a realized fracture, a fresh transverse osteotomy should be performed at the level of healthy, uninvolved bone.
A malleable retractor is placed medially after the soft tissues have been emancipated with a Cobb or similar elevator.
The psoas and adductors will be more easily tagged and released after the osteotomy, with retraction of the proximal femur segment laterally.
Care must be taken to avoid injury to the profundus femoral vessels.
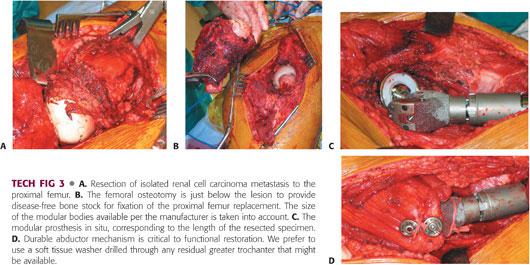
The modular endoprosthetic reconstruction length is determined by the planned length of femoral resection (TECH FIG 3C). Careful preoperative planning and familiarity with the incremental reconstruction levels of the selected implant are important to facilitate efficient reconstruction.
If no acetabular reconstruction is planned, a trial head is tested for size as usual.
Although a large stem diameter is preferable, overzealous reaming in this patient population is discouraged. Continual lavage and irrigation of the medullary contents is of critical importance.
A cement mantle of at least 1 mm is preferred; therefore, the stem diameter should be at least 2 mm smaller than the last reamed size for ease of introduction at cementation and to avoid monomer introduction into the circulatory system.
Taper and face-reaming of the proximal femur as described by the implant manufacturer may be necessary.
For cases of MBD, we prefer a longer, bowed stem. Cement precautions are mandatory.
Neck length is determined by preoperative planning and trial reduction.
After reduction of the trial, the capsule is pulled tight by stay sutures, and stability and length are assessed.
Anterior, posterior, and lateral stability should be evaluated. The sciatic nerve is evaluated.
Pulses should also be checked at this point. If they are diminished, that may indicate that the prosthesis is too long.
Orientation of the prosthesis is very important, with anteversion based on the sagittal plane created by the linea aspera. The prosthetic neck should be angled anteriorly 95 to 100 degrees off this plane.
The prosthesis is assembled as described by the manufacturer.
We strongly recommend against using cement that is too viscous.
A long laparoscopic-type suction device should be used continually throughout instrumentation of the femoral canal, and consideration should be given to venting if a long stem is to be deployed.
The canal should be brushed as well.
As the cement matures after prosthetic deployment, the surgeon must immediately and carefully confirm the selected version.
Soft tissue reconstruction is of paramount importance for a sound functional result.
The hip capsule should be purse-stringed about the prosthetic neck using a no. 5 polyfilament, nondissolving stitch.
Once repaired, it should not be possible to dislocate the hip anteriorly, posteriorly, or laterally.
The tagged psoas tendon may be sewn to the anterior capsule. Likewise, the external rotators may be sewn to the posterior capsule.
At this point, the sciatic nerve is again checked to make sure it is not compromised.
Numerous techniques have been described for reattaching the abductor mechanism to the implant. Manufacturers also describe various capture mechanisms. The surgeon must pay close attention to the reattachment mechanism because this is the limit of the functional reconstruction.
We prefer to use a soft tissue washer specific to the implant that can either be drilled through the residual trochanteric bone or harness the tendon itself (TECH FIG 3D).
The vastus lateralis muscle is repaired, as are the gluteus maximus and iliotibial band.
For metastatic cases, a drain is not mandatory unless the lesion is highly vascular (eg, renal cell and thyroid).
PEARLS AND PITFALLS | |
Potential cardiopulmonary collapse secondary to cementation of femoral components |
|
Extensive periacetabular bone loss |
|
Instability of proximal femoral replacements |
|
Abductor weakness in calcar- and proximal femoral–replacing reconstructions |
|
POSTOPERATIVE CARE
 In this patient population, all reconstruction must permit weight bearing as tolerated, with an assistive device as needed.
In this patient population, all reconstruction must permit weight bearing as tolerated, with an assistive device as needed.
 If a drain has been placed for metastatic cases, it should be discontinued within 72 hours.
If a drain has been placed for metastatic cases, it should be discontinued within 72 hours.
 Depending on the approach and the extent of the dissection, hip precautions should be implemented for 6 to 12 weeks.
Depending on the approach and the extent of the dissection, hip precautions should be implemented for 6 to 12 weeks.
OUTCOMES
 Patients with periacetabular lesions have 70% to 75% satisfactory pain relief and return to at least partial mobility.
Patients with periacetabular lesions have 70% to 75% satisfactory pain relief and return to at least partial mobility.
 Cemented total or hemiarthroplasty for femoral head, neck, and peritrochanteric lesions remains, in general, the procedure of choice in this patient population, with good to excellent outcomes relative to the omnipresent comorbidities.
Cemented total or hemiarthroplasty for femoral head, neck, and peritrochanteric lesions remains, in general, the procedure of choice in this patient population, with good to excellent outcomes relative to the omnipresent comorbidities.
COMPLICATIONS
 Periacetabular reconstructions are associated with complication rates of 20% to 30%.
Periacetabular reconstructions are associated with complication rates of 20% to 30%.
 Cemented femoral arthroplasty is not without inherent risk.
Cemented femoral arthroplasty is not without inherent risk.
Perioperative cardiopulmonary complications associated with cementing hip arthroplasty components are well described.12,14–16
Cement-associated desaturation and hypotension, pulmonary hypertension, cardiogenic shock, cardiac arrest, and intraoperative death are complications during femoral cementation and component placement secondary to canal pressurization.2,6,10
Cemented arthroplasty has been shown to be associated with more embolic events than noncemented arthroplasty, with higher IM pressures noted with cementation.12,13
Any factor that increases extrusion of femoral IM contents has been suggested to elevate the risk of cardiopulmonary embolic complications. In addition to cementation, this includes porous bone and the use of long-stem femoral implants. Long-stem components have been proposed to increase pressurization of the canal, producing more embolic events, with the rate of cardiopulmonary complications reported to be as high as 62%.6,11
Metastatic bone allows greater extrusion of emboli because of its permeative qualities and increased vascular supply. Thus, patients with MBD undergoing long-stem cemented femoral arthroplasty are at particularly high risk for cardiopulmonary compromise.
 Proximal femoral replacement is associated with complication rates as high as 28%.9
Proximal femoral replacement is associated with complication rates as high as 28%.9
Most experienced surgeons believe that proximal femoral replacement remains the best option for subtrochanteric involvement in proximal femur pathologic fractures secondary to MBD.
No better alternatives with lower risks than proximal femoral replacement exist for this difficult patient population.
REFERENCES
1. Alvi HM, Damron TA. Prophylactic stabilization for bone metastases, myeloma, or lymphoma: do we need to protect the entire bone? Clin Orthop Relat Res 2013;471(3):706–714.
2. Fallon KM, Fuller JG, Morley-Forster P. Fat embolization and fatal cardiac arrest during hip arthroplasty with methylmethacrylate. Can J Anesth 2001;48:626–629.
3. Forsberg HA, Wedin R, Bauer H. Which implant is best after failed treatment for pathologic femur fractures? Clin Orthop Relat Res 2013;471(3):735–740.
4. Hage WD, Aboulafia AJ, Aboulafia DM. Incidence, location and diagnostic evaluation of metastatic bone disease. Orthop Clin North Am 2000;31:515–528.
5. Harrington KD. The management of acetabular insufficiency secondary to metastatic malignant disease. J Bone Joint Surg Am 1981;63(4):653–664.
6. Herrenbruck T, Erickson EW, Damron TA, et al. Adverse clinical events during cemented long-stem femoral arthroplasty. Clin Orthop Relat Res 2002;395:154–163.
7. Jansen JA, van de Sande MA, Dijkstra PD. Poor long-term clinical results of saddle prosthesis after resection of periacetabular tumors. Clin Orthop Relat Res 2013;471(1):324–331.
8. Landis SH, Murray T, Bolden S, et al. Cancer statistics, 1998. CA Cancer J Clin 1998;48:6–29.
9. Papagelopoulos PJ, Galanis EC, Greipp PR, et al. Prosthetic hip replacement for pathologic or impending pathologic fractures in myeloma. Clin Orthop Relat Res 1997;341:192–205.
10. Parvizi J, Holiday AD, Ereth MH, et al. Sudden death during primary hip arthroplasty. Clin Orthop Relat Res 1999;369:39–48.
11. Patterson BM, Healey JH, Cornell CN, et al. Cardiac arrest during hip arthroplasty with a cemented long-stem component. J Bone Joint Surg Am 1991;73A:271–277.
12. Pitto RP, Koessler M, Draenert K. Prophylaxis of fat and bone marrow embolism in cemented total hip arthroplasty. Clin Orthop Relat Res 1998;355:23–34.
13. Pitto RP, Koessler M, Kuehle JW. Comparison of fixation of the femoral component without cement and fixation with use of a bone-vacuum cementing technique for the prevention of fat embolism during total hip arthroplasty. J Bone Joint Surg Am 1999;81A:831–843.
14. Randall RL, Aoki SK, Olson PR, et al. Complications of cemented long-stem hip arthroplasties in metastatic bone disease. Clin Orthop Relat Res 2006;443:287–295.
15. Randall RL, Hoang BH. Musculoskeletal oncology. In: Skinner HB, ed. Current Diagnosis and Treatment in Orthopedics, ed 4. New York: McGraw-Hill, 2006.
16. Ward WG, Spang J, Howe D. Metastatic disease of the femur: surgical management. Orthop Clin North Am 2000;31:633–645.
17. Weber KL, Lewis VO, Randall RL, et al. An approach to the management of the patient with metastatic bone disease. Instr Course Lect 2004;53:663–676.
< div class='tao-gold-member'>







