39 Hand therapy
Synopsis
 In optimal situations, hand therapists work closely with hand surgeons to maximize nonoperative and postoperative outcomes for patients with upper extremity conditions.
In optimal situations, hand therapists work closely with hand surgeons to maximize nonoperative and postoperative outcomes for patients with upper extremity conditions.
 Assessment in the area of impairment and body structures includes edema, wound, vascularity, pain, range of motion, strength, and sensibility.
Assessment in the area of impairment and body structures includes edema, wound, vascularity, pain, range of motion, strength, and sensibility.
 Following nerve injury and with chronic nerve compression, rehabilitation strategies should be directed not only to the distal recovery but also to re-establishing normal movement patterns and cortical remapping.
Following nerve injury and with chronic nerve compression, rehabilitation strategies should be directed not only to the distal recovery but also to re-establishing normal movement patterns and cortical remapping.
 The study of flexor tendon healing over time has demonstrated that early controlled motion is both beneficial to tendon healing and preferable to immobilization.
The study of flexor tendon healing over time has demonstrated that early controlled motion is both beneficial to tendon healing and preferable to immobilization.
 Hand fractures are challenging and as such require careful attention to bony integrity and maintenance of the flexor and extensor tendon-gliding systems.
Hand fractures are challenging and as such require careful attention to bony integrity and maintenance of the flexor and extensor tendon-gliding systems.
Introduction
Tenets of hand therapy
According to the Hand Therapy Certification Committee (www.htcc.org/about/index.cfm), the specialty of hand therapy is defined in the following manner:
Evaluative guidelines
A comprehensive evaluation provides valuable diagnostic and prognostic information to the plastic surgeon and hand therapist prior to intervention, and serves to inform clinical decision-making for management (nonoperative and postoperative) of patients with upper extremity conditions. In concert with the International Classification of Functioning, Disability and Health,1 hand therapists are well versed in the clinical assessment of patients in areas of impairment, activity, and participation.
Assessment in the area of impairment and body structures includes edema, wound, vascularity, pain, range of motion, strength, and sensibility. Edema is most commonly assessed with circumferential measurement. However, it is more accurately assessed using volumetric displacement with a reported test–retest reliability within ±3 mL.2 Wound assessment is characterized by both descriptive and objective components, including a detailed patient history and observation of wound size, color, drainage, odor, and temperature. Vascularity can be assessed both proximally and distally in the upper extremity using provocation maneuvers. In addition to the Allen’s test for circulation via the radial and ulnar arteries at the wrist, vascularity may be assessed more proximally using Adson’s maneuver at the subclavian artery; the costoclavicular maneuver and hyperabduction test assessed via the radial pulse; and the elevated arm stress test, as described by Roos.3–5 Pain, certainly a subjective and multifaceted construct, should be assessed before, during, and after therapeutic interventions. Numeric, verbal, and visual analog scales are commonly used to rate pain intensity and have been identified as having good construct validity.6 Simple measures which assess only pain intensity may be inadequate to capture and measure the multidimensional aspects of the pain. The McGill Pain Questionnaire and the Short-Form McGill Pain Questionnaire (SF-MPQ) are the most widely used pain questionnaires to assess the multidimensional qualities of the pain experience and overall have been shown to possess good to excellent psychometric properties.7–11 The SF-MPQ consists of a visual analog scale pain intensity, the present pain intensity index, and 15 pain adjectives (11 sensory, four affective) to calculate the pain-rating index.
Goniometric measurement is used to assess passive and active range of motion. While electrogoniometers and costly systems are available for clinical use, standard manual goniometers are easily portable and inexpensive (video 1). In a recent study, high intrarater reliability was found in multiple techniques to assess wrist range of motion, with a dorsal volar approach demonstrating the highest interrater reliability.12 Ellis and Bruton found composite finger flexion measurements to be as reliable as manual goniometry between raters, with manual goniometry demonstrating increased intrarater reliability for measurement of the proximal interphalangeal joint.13 Manual muscle testing is employed to isolate strength of individual muscles and often graded using the Medical Research Council (MRC) grade 0–5 classification.14 In a review, Cuthbert and Goodheart reported good evidence to support the use of manual muscle testing for patients with neuromusculoskeletal dysfunction.15 Sensory impairment is most often assessed using monofilaments for threshold testing (video 2) and/or two-point discrimination for innervation density.16–22 Using standardized techniques, good validity and reliability have been shown using these types of assessment tools.16,17,19,23–25 The Ten Test was introduced as a quick assessment of light touch sensation and has been shown to be a valid measure as compared to monofilament testing.26,27
Clinical assessment may also include grip and pinch strength testing.28–31 A calibrated, hand-held dynamometer is used to measure grip strength in a standard position of glenohumeral adduction, elbow flexion, and neutral forearm rotation.29 One trial has been identified as equally reliable to the best or average of three trials and elicits less patient discomfort.28 The influences of hand size and span have also been identified as factors to consider when selecting the grip handle position.32–34 Calibrated gauges are also used to measure both lateral and tip pinch.
A paradigm shift towards increased incorporation of qualitative and patient-reported data into outcomes assessment is strongly encouraged as a means of assessing participation.35 Clinical evaluation tools, such as range of motion, which assess physical impairments, have demonstrated poor validity and limited responsiveness as compared to patient self-report measures.36–39 Research has also shown a weak relationship between patient perception of health-related quality of life and ratings from healthcare providers.40 Patient perception of ability has been suggested as more valuable than functional evaluations.41 In addition, patient assessment of the importance of change in health status is preferable to that interpreted by the healthcare professional.42 Despite numerous tools and support in the literature, the incorporation of self-report outcome measures is inconsistent in clinical practice.43
The Disabilities of the Arm, Shoulder, and Hand (DASH), one of the most recognized self-report outcome measures for the upper extremity, was published as a joint effort of the American Academy of Orthopedic Surgery and the Institute for Work and Health.44 The DASH was created as a tool that would allow comparison of conditions throughout the upper extremity while considering it a single, functional unit.44,45 Careful development of this tool, including an extensive literature review and consideration of questions and attribution, has made it a popular tool in upper extremity research and clinical practice.
Rehabilitation following nerve injury/surgery
Compression neuropathies
Postoperative care following surgery for compression neuropathies is a balance between protecting the surgical repair site and maintaining neural mobility. To minimize adhesions and scarring, early motion is advocated. However the definition of “early motion” following release of an entrapment site is variable and is determined by the surgical procedure performed and surgeon preference.46,47 Compared to the historical reports in the literature, the tendency has been to decrease the length of time of immobilization and to use less restrictive postoperative dressings.25,48 Range-of-motion exercises are instituted and restoration of muscle balance is also a goal of postoperative care.
Carpal tunnel syndrome
Postoperative care
Historically, following release of the carpal tunnel, immobilization was used to protect the wound and to prevent bowstringing of the flexor tendons by restricting motion at the wrist. With recognition of the importance of early motion, the duration and type of immobilization following carpal tunnel release have substantially decreased. In our practice, a bulky dressing is used for restriction of wrist movement and for patient comfort in the immediate postoperative period. Following surgery, the patient is instructed in range-of-motion exercises for the fingers, elbow, and shoulder. Two days following surgery, the bulky dressing is removed and the patient is instructed in range-of-motion exercises for the fingers, wrist, elbow, and shoulder. For patient comfort, a splint in a wrist neutral position may be used at night. The sutures are removed 12–14 days following surgery. Return to work is dependent upon the type of job and specific patient factors. However at 4 weeks after surgery, patients should have full range of motion and may lift up to 2 lb (0.9 kg) and, by 2 months following surgery, full activity without restrictions is expected.48 Depending on the severity of nerve compression, the time to full sensory and motor recovery may vary.
Cubital tunnel syndrome
With failure of nonoperative treatment, there are numerous surgical procedures performed for the operative treatment of cubital tunnel syndrome, ranging from simple decompression, medial epicondylectomy to various types of anterior transposition.48 With each procedure, there are numerous protocols for the postoperative management and many vary depending upon surgeon preference. In general, early motion is recommended to minimize adhesions and scarring of the ulnar nerve and surrounding soft tissue.
Postoperative care
There is wide variability in the duration of immobilization following surgery for cubital tunnel syndrome and this depends upon the operative procedure performed and surgeon preference. The period of immobilization is increased for those procedures that involve more complex release of the soft tissue surrounding the ulnar nerve (i.e., least immobilization for simple decompression). In most cases, the postoperative dressing may be removed 2–3 days after surgery and then a postoperative method of immobilization may be used if required. For patient comfort, a sling may be used at night to restrict upper extremity motion while the patient is sleeping. The postoperative care following a transmuscular transposition will be described.48 In our experience, patients may begin range-of-motion exercises (fingers, wrist, forearm, elbow, and shoulder) when the dressing is removed at postoperative day 2 or 3. Patients are cautioned to perform these exercises slowly and initially to avoid full forearm supination combined with full elbow extension because this motion will place the most stress on the soft-tissue repair. Initially elbow extension is performed with the forearm in pronation and then, as a separate exercise, forearm supination is performed with the elbow flexed at 90°. When the patient is able to move comfortably into full supination, forearm supination is combined with elbow extension. A sling is used at night for patient comfort to avoid rapid elbow extension and may be discontinued when the patient has achieved full elbow extension with full forearm supination. The emphasis in the early postoperative period is to regain full range of motion and it is anticipated that this is achieved within the first 2–3 weeks following surgery. If the patient appears to be progressing slowly, more structured hand therapy is recommended. Strengthening exercises are instituted 1 month following surgery and full activity is permitted at 8 weeks.
Concomitant soft-tissue muscle imbalances and other sites of nerve compression in the upper extremity may require rehabilitation therapy to achieve full recovery. Some patients may also have restricted neural excursion, cervicoscapular discomfort, and/or rotator cuff tendinitis associated with muscle imbalances in this region. Specific exercises to address these more proximal soft-tissue and neural structures will help to ameliorate these types of symptoms in patients with more diffuse upper extremity discomfort, paresthesia, and/or numbness.49–56
Nerve repair
Early postoperative care
In the immediate postoperative period, the primary goal is to protect the nerve repair site. Initially, a bulky dressing is used and, if required by the operative procedure, more rigid support can be utilized. The patient is instructed in edema control and range-of-motion exercises for the joints that are not immobilized by the bulky operative dressing. The initial dressing is removed within a few days following surgery. Immobilization is continued with the use of a custom-made or prefabricated splint or, in some cases, a sling or shoulder immobilizer may provide sufficient support to restrict forces on the repair site. Nerve repairs compared to nerve grafts or transfers are typically immobilized for longer periods of time and in some cases up to 3 weeks. The timing of immobilization will depend upon the location of the nerve repaired and the amount of nerve mobility. In some cases, such as with digital nerve injuries, some have advocated no immobilization after the initial operative dressing has been removed.57 With a nerve graft or transfer, there is extra nerve length at the repair site and lack of tension associated with these reconstructions and, as such, these types of reconstruction require less rigid immobilization and may be mobilized earlier (7–14 days) compared to nerve repairs. However, other soft tissues (tendons, muscles, or ligaments) which may have been released or repaired in the operative procedure may prolong the period of immobilization. For example, repair of the pectoralis minor with exploration of the brachial plexus may require 4 weeks of immobilization in internal shoulder rotation using a shoulder immobilizer to avoid tension on the soft-tissue repair or, with acute injuries that may involve a tendon repair, a tendon rehabilitation protocol would be followed.
Early postoperative pain resulting from the operative procedure may be controlled with analgesics, edema control, early motion, and hand therapy. However ongoing neuropathic pain may negatively impact outcome and therefore early intervention is recommended.58,59 Neuropathic pain following trauma may require a comprehensive multidisciplinary approach and as such referral to a pain management team may be warranted.
The use of electrical muscle stimulation is often advocated following nerve injury to prevent muscle degeneration and to protect the muscle fiber until regeneration occurs. However, the literature does not present strong support for the use of this modality following nerve injury.60,61 Historically, the rationale for electrical stimulation of denervated muscles has been to retain integrity of the muscle fibers while waiting for muscle reinnervation. Studies using animal models of denervated muscles and electrodes implanted directly into the muscle have shown preservation of some normal muscle properties.62,63 However, no efficacy trials have provided evidence of these benefits in humans. More recent evidence presented in the literature suggests that longer durations of axotomy and Schwann cell denervation are predictive of poor functional outcome.64–66 Low-frequency stimulation of the nerve following nerve transection and repair in animal models has shown an increase in axonal regeneration into the appropriate neural pathway.64,67 In patients after carpal tunnel release, 1 hour of low-frequency nerve stimulation resulted in significantly faster motor reinnervation.67 This evidence provides the impetus for future investigation into the benefits of direct nerve stimulation to improve recovery following a motor nerve injury. However, the use of direct current stimulation for denervated muscles has not been shown to be efficacious in humans and as such is not advocated by the authors.
Sensory re-education
Following sensory nerve reconstruction, sensory re-education is commonly included in the rehabilitation program to improve hand sensibility and function. These strategies have been used to maximize sensory recovery and also to decrease pain and for desensitization related to allodynia and/or hyperalgesia.68–73 Desensitization exercises using different textures and vibration techniques may be used to decrease allodynia and/or hyperalgesia. The concept of vibration for desensitization relates to the gate control theory of pain and stimulation of large A-beta fibers with vibration.74 The use of small-amplitude vibration for desensitization is most tolerated by patients and may be obtained by using an electric shaver with the cap on it. This will provide an easy technique for the patient to continue this exercise in a home program. With decreasing sensitivity, the area of allodynia may be challenged with different types of texture to promote sensory re-education and appropriate cortical mapping.
With evidence of reinnervation of the sensory receptors, sensory re-education is advocated.69 This typically includes a structured program with an emphasis on textures, localization, and discriminatory tasks.69 The exercises are progressed to challenge the sensory system as the patient’s sensation improves, beginning with varying textures, localization exercises, and then discriminatory tasks. However, strategies to enhance and promote cortical remapping have been also advocated in the earlier phases following surgery. Rosen and Lundborg have described re-education programs using mirror imagery, visuotactile training, and audiotactile interaction to enhance the functional reorganization of the sensory cortex in the early phases of recovery before there is evidence of sensory receptor reinnervation.75
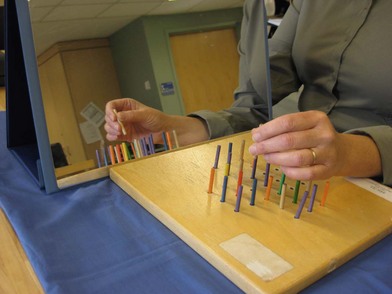
Mirror visual feedback has been extensively used for treatment in patients following stroke and with amputation injuries and has been more recently reported for use in patients with pain syndromes or nerve injury.76,77 The concept of mirror visual feedback is that patients observe their reflected image in the mirror, which would appear as the contralateral limb (Fig. 39.1) and thus activate the brain. Patients may perform specific exercises or movements with the unaffected hand or extremity and in the mirror it would appear as normal movement to the affected hand or extremity. With improvement in sensory function or decreased pain, more complex activities may be introduced.
Motor re-education
The goals of treatment for motor function are directed towards re-establishment of normal movement patterns and muscle balance. Following nerve injury, motor re-education is not as frequently advocated compared to sensory re-education. Alterations in cortical mapping and motor patterns occur following injury to a motor nerve.78–80 With reinnervation of the denervated muscles, muscle imbalances will remain due to weakness of the reinnervated muscles compared to the uninjured muscles and also compensatory movement patterns that have performed since the injury. Particularly in patients with brachial plexus injuries, the period of time until muscle reinnervation occurs may be prolonged (12–18 months) and abnormal movement patterns are established. Therefore motor re-education will provide the opportunity to focus on appropriate muscle recruitment and muscle balance. Even in patients with only sensory loss following median nerve injury, there will be alteration of the motor pattern due to compensation in movements as a result of numbness to the sensory distribution of the median nerve. Therefore, the emphasis should be on restoration of the integration of motor and sensory re-education following nerve injury. Sensorimotor control to perform upper extremity tasks requires the coordination of both the sensory and motor systems.81 Alteration in either motor or sensory function as a result of nerve injury will impact sensorimotor control in the upper extremity. Improvement in hand function may occur with increased use of the hand, but progress may be enhanced with the use of activity-based sensorimotor retraining, emphasis on control, and appropriate feedback learning. As with any new task learning, repetition and feedback are essential and therefore patient education and an effective home program should be emphasized.
Nerve transfers
Historically, motor nerve transfers were used as a salvage procedure when nerve repair or graft was not possible due to avulsion of the proximal nerve source, and there was limited potential for excellent outcome. However, the utilization of nerve transfers has increased, particularly with proximal nerve injuries, where recovery is limited due to the distance from the injury to target muscle fibers and prolonged time for reinnervation.82–84 Because a nerve transfer can provide a closer innervation source, the distance and time for reinnervation are shorter and the results following these types of transfer have been very encouraging.85–93 However, optimal results depend not only on the number of regenerating motor axons and reinnervation of muscle fibers but also on cortical plasticity and remapping. Previously established motor patterns and cortical maps are no longer relevant because a new proximal nerve source is used with a nerve transfer. Studies have evaluated cortical mapping following nerve transfers, muscle transfers, and toe-to-thumb transfers.78–80,94–97 Collectively, these studies have illustrated shifts in the motor cortex following transfers and the importance of effective relearning to optimize outcome. Therefore rehabilitation programs should include not only range-of-motion and strengthening exercises to regain muscle balance, but also motor re-education to promote normal movement patterns and cortical mapping.98,99
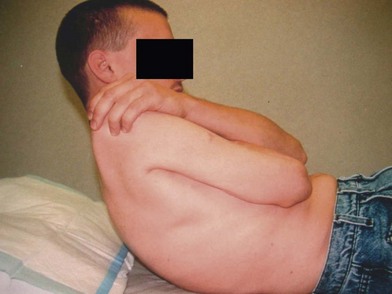
In patients with brachial plexus avulsion injuries, an intercostal to musculocutaneous nerve transfer is often performed to restore elbow flexion. Postoperative education often includes deep-breathing exercises to provide a stimulus to the intercostal nerves for contraction of the biceps and brachialis muscles, which are innervated via the musculocutaneous nerve. While deep breathing does initiate contraction of the newly innervated elbow flexors, it is difficult to provide a prolonged contraction for re-education and strengthening. The abdominal muscles and intercostal muscles are activated with core abdominal exercises. Therefore core-strengthening exercises will also provide contraction of the biceps and brachialis muscles in patients who have had an intercostal-to-musculocutaneous nerve transfer. The muscle activity in the elbow flexors associated with trunk flexion has been shown by Chalidapong and colleagues.100 Core exercises which use the abdominal and intercostal muscles may begin early in the rehabilitation stages (Fig. 39.2) and with early reinnervation will recruit the muscle fibers in the brachialis and biceps.
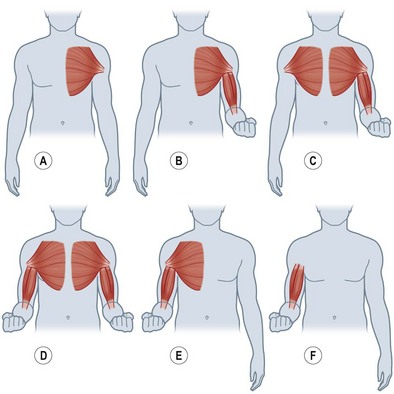
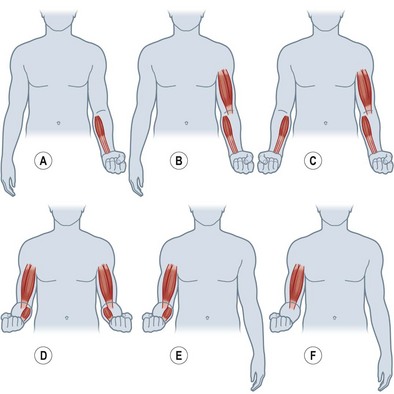
More recent descriptions of nerve transfers to restore elbow flexion have included donor pectoral nerves and fascicles from the median and ulnar nerves.87,93 To assist with the retraining following these types of nerve transfer, re-education may begin preoperatively with education on the unaffected extremity and contraction of the muscles from the donor nerve and recipient muscle. In this way, the patient may practice co-contraction of these muscle groups to understand better the re-education that will be required with reinnervation of the denervated muscles. For a medial pectoral-to-musculocutaneous nerve transfer, the patient will simultaneously contract the pectoral and the biceps (and brachialis if included) muscles (Fig. 39.3). This is initially done bilaterally and then only to the injured side. Using place-and-hold positioning in 90° of elbow flexion, an isometric contraction is performed. Once the patient is able to hold this contraction against gravity, the degree of flexion may be altered and progressed through a full range of motion. A double fascicular transfer from the median and ulnar nerves to restore elbow flexion will require contraction of the wrist flexors to initiate contraction of the biceps and brachialis muscles (Fig. 39.4).
To restore innervation to the ulnar nerve intrinsic muscles, an anterior interosseous nerve (AIN) to deep motor branch of the ulnar nerve transfer may be performed.88 With an AIN to deep motor branch of the ulnar nerve transfer, attempts by the patient to pinch must be combined with forearm pronation to recruit the newly innervated intrinsic muscles from the AIN proximal source (Fig. 39.5). With motor retraining, cortical remapping, and relearned motor patterns, the patient will be able to complete the ulnar motor nerve actions independent of the action of forearm pronation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree