Chapter 81 Growth Factors and Other New Methods for Graft-Healing Enhancement
Anterior cruciate ligament (ACL) reconstruction using tendon autograft has been greatly improved over the last 2 decades.1 In ACL reconstruction, however, the strength of the grafted tendon is reduced in the early phase after surgery, and then it gradually increases.2–4 A problem is that this graft remodeling occurs very slowly.5 The slow graft maturation may result in graft failure or elongation during the postoperative rehabilitation period due to unknown causes. In addition, a firm attachment of a tendon graft to the bone is a significant factor for success in ACL reconstruction. In procedures using a hamstring tendon graft, however, the anchoring strength of the soft tissue in a bone tunnel is the weakest in the femur–graft–tibia complex in the early phase after surgery.6 To improve these problems after ACL reconstruction in the near future, we should try to develop a new strategy to accelerate the intraarticular and intraosseous remodeling of the tendon graft. This may enable more aggressive rehabilitation and an earlier return to rigorous sports for patients with ACL reconstruction. In this chapter, the authors will review recent experimental studies that are intended to enhance intraarticular and intraosseous graft healing after ACL reconstruction using growth factors, gene therapy, and cell-based therapy.
Basic Knowledge to Enhance the Graft Remodeling in Anterior Cruciate Ligament Reconstruction
In ACL reconstruction, intrinsic fibroblasts of the tendon graft are necrotized immediately after transplantation, and then numerous extrinsic fibroblasts infiltrate the graft with revascularization.7,8 However, it is possible that the cell infiltration into a core portion of the graft occasionally occurs very slowly. Delay et al5 reported a clinical case in which the core portion of the patellar tendon graft still remained necrotic even at the 18-month period after ligament reconstruction. On the other hand, biomechanically, the mechanical properties of the graft deteriorate in the early phase after transplantation and then are very gradually restored over a long period.2–4
Concerning the graft deterioration mechanism, the fibroblast necrosis itself does not deteriorate the mechanical properties of the tendon matrix, but extrinsic fibroblasts proliferating after the necrosis reduce the strength properties.9 In the extrinsic fibroblasts, type III collagen is overexpressed even under physiological stress in areas where extrinsic fibroblasts infiltrate.10 In the matrix of the autograft after transplantation, ultrastructurally, fibrils having a diameter less than 90 nm predominantly increase in the graft matrix, and these fibrils with small diameters still remain predominant at the 4-year period after surgery.11 Such ultrastructural changes due to type III collagen production are considered to be one of the causes of mechanical deterioration of autografts.
What molecular mechanisms control the autograft remodeling? In a rabbit ACL reconstruction model, vascular endothelial growth factor (VEGF) is overexpressed in the extrinsic fibroblasts at 2 weeks after graft implantation, followed by vascular formation at 3 weeks.12 This fact shows that revascularization in the graft is induced by VEGF produced in the fibroblasts. On the other hand, basic fibroblast growth factor, transforming growth factor (TGF)-β, and platelet-derived growth factor (PDGF) are overexpressed in the autogenous patellar tendon graft used to reconstruct the ACL in the canine model, reaching their greatest expression 3 weeks after implantation.13 This fact suggests that a complex growth factor network controls the fibroblasts, resulting in remodeling of the graft matrix,14,15 and implies that control of the fibroblasts using growth factors is a potential strategy to accelerate the graft remodeling after ACL reconstruction.
Enhancement of Graft Healing with Growth Factors
Intraarticular Healing
PDGF-BB
It has been known that PDGF-BB enhances proliferation and migration of ligament fibroblasts in vitro.16,17 In addition, an in vivo rabbit study showed that the strong expression of PDGF correlates with the observed increased cellularity around the wound site in the medial collateral ligament (MCL), suggesting potent mitogenic and chemotactic properties of PDGF in vivo.18 Concerning the in vivo effect of application of PDGF-BB on ligamentous tissues, Woo et al19 and Hildebrand et al20 described that 20-μg PDGF-BB alone is the most effective agent to enhance the extraarticular MCL healing in the rabbit. Regarding intraarticular ACL reconstruction, Weiler et al21 applied PDGF-BB of approximately 60 μg using coated sutures as a vehicle on the flexor tendon autograft in a sheep ACL reconstruction model. They showed that the PDGF-BB application significantly increased the load to failure and vascular density of the graft at 6 weeks after ACL reconstruction, although they found no significant effects at 24 weeks. However, Nagumo et al22 investigated the effect of PDGF-BB using fibrin sealant as a carrier on the in situ frozen-thawed rabbit ACL, an idealized intraarticular autograft model.23–25 They reported that an application of 4-μg PDGF-BB did not significantly affect the mechanical properties of the frozen-thawed ACL at 12 weeks. Hildebrand et al20 suggested that the dose is critical to evaluate the effect of growth factors on ligament healing. Application of a high dose of PDGF-BB appears to be effective for MCL healing. However, the effect of PDGF-BB in ACL reconstruction is controversial.
VEGF
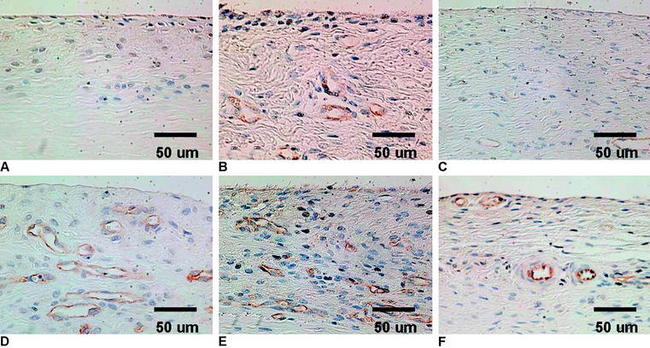
VEGF is a potent mediator of angiogenesis, which involves activation, migration, and proliferation of endothelial cells, in various pathological conditions.26 A rabbit study showed that extrinsic cells newly proliferating in the necrotized tendon graft express VEGF at 3 weeks after surgery, when the revascularization does not occur.12 This finding suggests the high possibility that an application of VEGF to the necrotized tendon graft enhances angiogenesis in the graft and accelerates remodeling of the graft. Ju et al27 histologically and mechanically examined the effect of an application of 30-μg VEGF to the in situ frozen-thawed rabbit ACL. The application of VEGF significantly enhanced vascular endothelial cell infiltration and revascularization in the ACL at 3 and 12 weeks, respectively (Fig. 81-1). On the other hand, the application provided no significant effect on the mechanical properties of the ACL at 12 weeks, although the mechanical properties of the in situ frozen-thawed rabbit ACL were significantly weaker than those of the normal ACL at 12 weeks. Thus VEGF has a potential to be used as a treatment to enhance only revascularization of the autograft after ACL reconstruction surgery.
TGF-β and EGF
A number of in vitro studies have shown that TGF-β enhances collagen and noncollagenous protein synthesis in fibroblasts.28–30 EGF also stimulates fibroblast proliferation in vitro.31 A combined application of these two growth factors enhances these effects.32 Sakai et al25 examined in vivo effects of an application of TGF-β and EGF on the in situ frozen-thawed ACL, using fibrin sealant as a vehicle. They found that a combined application of 4-ng TGF-β and 100-ng EGF significantly inhibited the natural deterioration that occurred in this autograft model with significant reduction of the water content and significant changes of the ultrastructural profile. Azuma et al33 investigated the effect of the timing of this combined application on the same rabbit model. They reported that the effect was significantly greater when 4-ng TGF-β and 100-ng EGF were applied at 3 weeks than when they were applied at 0 and 6 weeks. This study suggested that the timing is critical in application of these growth factors. Recently, Nagumo et al22 distinguished between the effect of 4-ng TGF-β and the effect of 100-ng EGF on the autograft model. According to them, the effect of 100-ng EGF was not significant, but the effect of 4-ng TGF-β was significant. Concerning an intraarticular application of TGF-β and EGF on the bone–patellar tendon–bone (BPTB) autograft in a canine ACL reconstruction model, Yasuda et al34
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree