30 Growth considerations in pediatric upper extremity trauma and reconstruction
Synopsis
 Skeletal growth is possible because of the presence of an active physis.
Skeletal growth is possible because of the presence of an active physis.
 The physis is physiologically regulated by several factors and can be hindered by congenital conditions (chondrodysplasias), direct damage, or interruption of its blood supply.
The physis is physiologically regulated by several factors and can be hindered by congenital conditions (chondrodysplasias), direct damage, or interruption of its blood supply.
 When the physis is damaged, therapeutic options include completion of epiphyseal arrest, resection of epiphyseal bar, and epiphyseal distraction.
When the physis is damaged, therapeutic options include completion of epiphyseal arrest, resection of epiphyseal bar, and epiphyseal distraction.
 Where there is involvement of the whole epiphysis, autologous epiphyseal transfer may achieve the dual goals of restoring joint function and growth potential.
Where there is involvement of the whole epiphysis, autologous epiphyseal transfer may achieve the dual goals of restoring joint function and growth potential.
Introduction
• The growth plate is a temporary anatomical entity, which allows axial growth in long bones. Once skeletal maturity is approached, the function of the growth plate gradually decreases and eventually stops.
• Trauma, infection, irradiation, thermal injury, tumor, and congenital disorders may affect the growth plate and interfere with the growth process.
• Any damage to the growth plate in a skeletally immature individual leads to growth disturbance with deformity and/or length discrepancy.
• Goals of the surgical treatment should be restoration of the physiological growth, prevention of angular deformities, and correction of established deviation of bones and joints.
Basic science/disease process
Anatomy and physiology of the epiphyseal growth plate
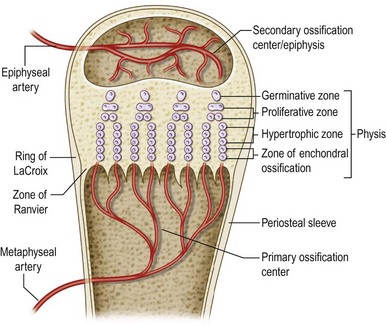
The physis (also known as growth plate, epiphyseal plate, epiphyseal growth plate, epiphyseal cartilage) is a highly specialized and organized cartilaginous structure derived from the mesoderm. It develops in the bone bud, secondary to the primary ossification centers (metaphysis) and is responsible for longitudinal and circumferential bone growth.1 The physis must be distinguished from the epiphysis, or secondary ossification center (Fig. 30.1).
The physis consists of proliferating chondrocytes surrounded by synthesized extracellular matrix. The extracellular matrix is composed of water, collagen fibrils (mainly types II, IX, X, and XI) and proteoglycans (aggrecan, decorin, annexin II, V, and VI) arranged to form a sort of sponge with very small pores.2 This arrangement confers peculiar mechanical properties that permit the physis to be “hard” when an axial load is applied rapidly (a jumping child) or “soft” when deformed slowly (when chondrocytes secrete new extracellular matrix).3
The physis is traditionally divided into horizontal zones of chondrocytes at different stages of maturation (Fig. 30.1). The resting zone (reserve zone or germinal matrix), immediately adjacent to the epiphysis, contains small, uniform, irregularly scattered chondrocytes, also referred to as stem cells, with low rates of proliferation but rich in storage materials (lipids and cytoplasmic vacuoles) for later growth.4,5 The resting zone is responsible for protein synthesis and for maintaining a germinal structure. Injury to this layer results in cessation of growth.
When chondrocytes enter into the proliferative zone they undergo rapid duplication, increase the synthesis of collagen, in particular types II and XI,4 and become flat and well organized into longitudinal columns. Mitotic activity is present only at the base of these columns. This layer is responsible for longitudinal growth of the bone via active cell division. These first two zones have an abundant extracellular matrix that confers a great deal of mechanical strength, in particular in response to shear forces.
The presence of provisional calcification4–6 confers shear resistance to the lower hypertrophic zone. In contrast, the upper hypertrophic zone containing scant extracellular matrix is the weakest portion of the physis and it is here that most injury or alteration to the physis occurs.7–9
The periphysis is a fibrochondroosseous structure that surrounds the physis of tubular bones and plays an important role in skeletal development10 (Fig. 30.1): (1) it allows gradual circumferential growth of the physis; (2) it continues to maintain its transverse diameter11; and (3) it is critical to the overall stability of the growth plate and to support for the physis bone–cartilage junction.11–13
The part of the periphysis adjacent to the epiphysis is a wedge-shaped group of germinal cells known as the zone of Ranvier; while the part adjacent to the metaphysis is known as the ring of LaCroix. Histologically, the Ranvier and LaCroix zones are a single structure (Fig. 30.1).
Chondrocyte proliferation and differentiation in the physis, and therefore longitudinal skeletal growth, are regulated on both a systemic and local level.14 A number of endocrine, paracrine, and autocrine factors and their respective receptors are involved in this process. Amongst these, the parathyroid hormone-related protein (PTHrP) and the Indian hedgehog (Ihh) play a prominent role in skeletal maturation.1,15,16 There is also mechanical control of growth, which is important in pediatric orthopedic surgery because it is the basis of the widely used epiphysiodesis and epiphyseal distraction procedures.3
Vascular anatomy of the growth plate
The epiphyseal and physeal cartilage vascular anatomy in newborn and early postnatal life is integral to long-bone development and differs from later postnatal periods. In late fetal and early postnatal life epiphyseal cartilage canals (or transphyseal vessels) are seen passing through the physeal cartilage and communicating with the metaphyseal marrow. Such vessels play an active role in forming the secondary ossification centers17,18 but their primary earlier function is to provide nutrition by diffusion to epiphyseal chondrocytes.
The transphyseal vessels are constantly obliterated several months after birth. Once the obliteration has occurred, the physeal cartilage becomes an avascular structure supplied by the epiphyseal vessels, by diffusion, and by the metaphyseal vessels invading the lowermost regions of the hypertrophic zone.19,20
The main vascular supply to the germinal zone is from the epiphyseal vessels. Two types of epiphyseal vascularization are described.21 Type A epiphyses are almost entirely covered by articular cartilage, and the epiphyseal vessels enter the epiphysis after traversing the perichondrium. With this anatomical configuration, the blood supply to the epiphysis, and consequently to the germinal zone, is susceptible to damage if the epiphysis is separated from metaphysis. Type B epiphyses are only partially covered by articular cartilage. Their blood supply enters from the epiphyseal side and is protected from vascular injury during separation. The proximal femur and proximal radius are the only two examples of type A epiphyses.
Growth plate closure and skeletal age assessment during puberty
It is generally assumed that long-bone physes close at 14 years of skeletal age in females and at 16 years in males while the axial skeleton completes its development later.22–24 In 50% of normal children and adolescents, skeletal age does not differ from chronological age.25 Prediction of limb length discrepancy and final standing height as well as decisions regarding when to perform an epiphysiodesis are first subjected to determination of skeletal age.
A complication unique to physeal injuries is growth disturbance. Once damage to the physis has occurred, the predicted amount of growth remaining from that specific physis must be determined to plan the treatment. This can be accomplished by determining the skeletal age of the patient and then using information on physeal growth rates and patterns assembled by Green and Anderson.26–30
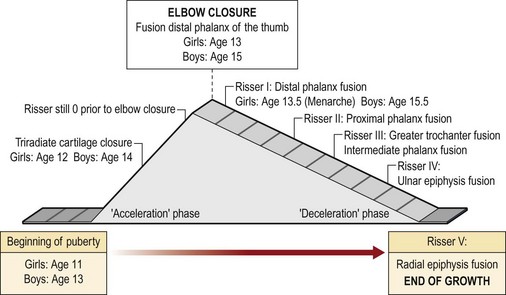
Treatment decisions are often made during puberty. Puberty is the period of time in the growing individual characterized by a significant increase in growth rate.31,32 In girls, puberty starts at 11 years and ends at 13 years of skeletal age; in boys, it starts and ends 2 years later (13 years and 15 years of skeletal age).25 Peak height growth and Tanner stage 233 signal the beginning of puberty. The 2 years of pubertal growth spurt are known as acceleration phase followed by a deceleration phase that continues until skeletal maturity.
The radiographic markers currently used to assess skeletal age are all based on the appearance of ossification centers in specific skeletal segments at specific times. Several age assessment methods are based on the use of anteroposterior radiographs of the left hand and wrist.34–37 Other methods use left elbow radiographs.25,38 The axial skeleton can also be used to determine the skeletal age: Risser sign39 is based on the appearance of the left iliac apophysis of the pelvis. However, other radiographs can be used to assess skeletal age.25
Different assessment methods become helpful at different ages. The Greulich and Pyle atlas34 is probably the most common and widely used method to assess skeletal age. This technique, however, has some limitations, especially during puberty because it does not make it possible to assess skeletal age at 6-month intervals during the 2 years of peak growth rate.25 On the other hand, during the pubertal acceleration phase, the elbow, at the level of the distal epiphysis ossification centers and the olecranon apophysis, undergoes peculiar and identifiable morphological changes every 6 months25,38 (Figs 30.2 and 30.3). Elbow ossification centers always appear in a determined sequence (the mnemonic is CRITOE: capitellum, radial head, internal (medial) epicondyle, trochlea, olecranon, external (lateral) epicondyle). The ages of appearance as a general guide, are 1–3–5–7–9–11 years.
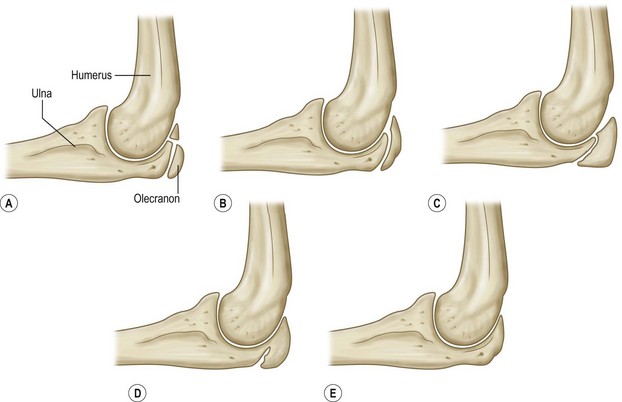
Fig. 30.2 Olecranon maturation according to Dimeglio et al.,35 with significant changes occurring every 6 months. (A) two ossification centers (girls 11 years; boys 13 years); (B) half-moon shape (girls 11.5 years; boys 13.5 years); (C) rectangular shape (girls 12 years; boys 14 years); (D) beginning of fusion (girls 12.5 years; boys 14.5 years); and (E) complete fusion (girls 13 years; boys 15 years).
During the acceleration phase, the skeletal age is best assessed with X-rays of the left hand and left elbow.40–43 In the deceleration phase, the growth rate decreases significantly. The remaining growth in both boys and girls is around 6 cm, of which 4.5 cm belong to the trunk and skull. During this phase, the most useful tools for skeletal age assessment are again the left hand and the Risser stage.
Diagnosis/patient presentation
Conditions affecting the growth plate
A complication unique to physeal injuries is growth disturbance. Trauma is the most common cause of physeal injury and growth disturbance, but physeal growth arrest may as well occur after infection, tumor, irradiation, thermal injury, laser beam exposure, and sequelae of Blount’s disease (growth disorder with bowing of the tibia).44–47
Trauma
Incidence and distribution in the upper extremity
Physeal injuries represent 15–30% of all fracture in children48–52 and frequently involve the upper extremities.51,53 The incidence varies with age, with a peak in adolescence.51,53,54 The upper hypertrophic zone, just above the area of provisional calcification, is the weakest layer of the physis and where most injuries to the physis occur.7–9 This observation implies that, after most injuries, the germinal layer of the physis remains intact and attached to the epiphysis. Consequent normal growth should resume unless insult to the blood supply of the germinal layer or development of a “bony bridge” across the injured physis occur.
Classification of physeal fractures
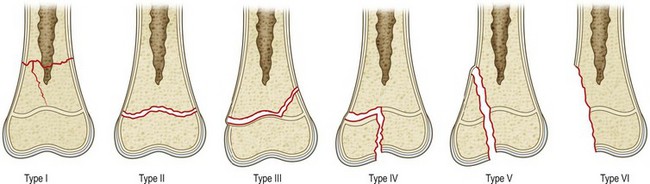
Several classification systems for physeal injuries have been described.55–61 The Salter and Harris classification (Fig. 30.4)62 is by far the most widely used system. This classification helps to distinguish different types of fractures and provides prognostic information as well. In Salter–Harris type I (Fig. 30.4), the injury is a separation of the epiphysis from the metaphysis. It occurs entirely through the physis and therefore the surrounding bone is not involved. It is rare and tends to be seen in infants or pathologic fractures, such those secondary to rickets or scurvy. Because the germinal layer remains with the epiphysis, growth is not hindered unless blood supply is interrupted (traumatic separation of the proximal femoral epiphysis). Often, X-rays of a child with a type 1 Salter–Harris fracture will appear normal. Healing of type I fractures tends to be rapid and complications are rare.
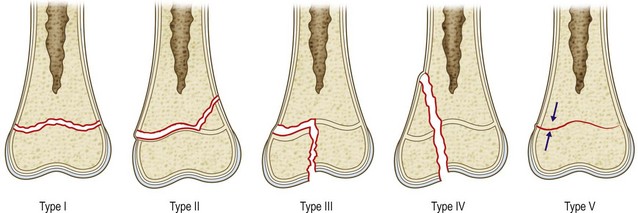
Fig. 30.4 Salter–Harris classification of physeal fractures.
(Redrawn after Salter RB, Harris R. Injuries involving the epiphyseal plate. J Bone Joint Surg Am. 1963;45:587–621.)
In Salter–Harris type II injury (Fig. 30.4), the fracture extends along the hypertrophic zone of the physis but then it continues and exits through the metaphysis. This is the most common type of growth plate fracture, and tends to occur in older children. The epiphyseal fragment contains the entire germinal layer as well as a metaphyseal fragment, known as Thurston Holland’s sign. The periosteum on the side of the metaphyseal fragment is usually intact and provides stability after reduction. Growth disturbance is uncommon because the germinal layer remains intact.
In Salter–Harris type III injury (Fig. 30.4), the fracture starts through the hypertrophic zone and exits through the epiphysis. These injuries also tend to affect older children. By definition, type III fractures cross the germinal layer and are usually intra-articular. Consequently they raise concerns about growth and, when displaced, they require anatomic, and often open, reduction.
In Salter–Harris type IV injury (Fig. 30.4), the fracture starts from the metaphysis and then extends through the physis and into the epiphysis. By definition these fractures disturb the germinal layer and are usually intra-articular. Consequently, these injuries may impair normal growth and affect articular congruency. In type IV injuries, an anatomic reduction is mandatory in order to prevent osseous bridging across the physis and to restore the articular surface.
A Salter–Harris type V injury (Fig. 30.4) occurs when the physis is crushed from a pure compression force. It is so rare that some authors question whether such an injury exists.63 For those authors who have reported on this injury,62,64 it carries the most concerning prognosis as the growth disturbance is almost a rule. It often requires later surgical treatment to restore limb length and alignment.
Despite being widely used, the Salter–Harris classification excludes few physeal injuries: the Rang’s type VI epiphyseal injury59,60 which represents an injury to the perichondral ring; and two additional injuries described in the Peterson classification system (Fig. 30.5),54 which is very similar to the Salter–Harris scheme. The Peterson type I fracture is a transverse fracture of the metaphysis that extends longitudinally into the physis. Clinically this type of fracture is commonly seen in the distal radius. The Peterson VI fracture is an open injury associated with loss of the physis.
Treatment of physeal fractures
The age of the patient, the location and displacement of the fracture, and the time elapsed since the injury must be all taken into consideration when assessing a nonanatomic reduction. Although animal studies have not shown that delay in reduction produces a growth disturbance,65 the recommendation is to accept any displacement in type I and II injuries after 7–10 days9,66 and rather plan an osteotomy later. As a general rule the remodeling potential is inversely proportionate to the age of the patient and is also related to the location and type of injury. Consequently, a greater deformity can be accepted in younger children.
Tumor
Bone sarcoma involving the epiphysis
Most of the common primary malignant bone tumors occur in patients aged under 30 years.67 Typically osteosarcoma and Ewing sarcoma are found in adolescents and young adults, but they may also be diagnosed during infancy. The current approach to systemic therapy67includes preoperative (neoadjuvant) chemotherapy and postoperative (adjuvant) chemotherapy for both osteosarcoma and Ewing sarcoma. Radiotherapy is indicated only in Ewing sarcoma and it may be the only local treatment in cases of tumor in unfavorable locations, such as spine and pelvis, and in all cases where radical surgery is unlikely to be successful. Postoperative radiotherapy is indicated in all cases of intralesional resections with the aim of reducing the risk of local recurrence.
In the past, the growth plate was thought to be a biologic barrier to bone tumor invasion68–70; unfortunately this theory has not proven to be valid71–73 (Fig. 30.6). The current view is that invasion of the physis must always be suspected when the tumor is located in the proximity of the growth plate and all patients should be investigated with magnetic resonance imaging (MRI). In the event of epiphyseal involvement, the oncological resection must include the epiphysis and the only possible reconstructive option for such a defect in the pediatric age is autologous epiphyseal transplant.
Patient selection
When the physis is damaged and deformity results or is foreseen, several treatment options are available. The best treatment for a growth plate injury depends on the individual situation. Although physeal injuries are common, problems arising are rare, occurring in only 1–10% of all physeal fractures.9,51,54 Comminuted fractures, high-energy injuries, and physeal injuries that cross the germinal layer are more prone to result in physeal arrest with subsequent growth disturbance. Physeal injuries are most common in adolescents close to skeletal maturity. In these individuals, the remaining growth is limited.9,51,54 Consequently, even when it occurs, physeal arrest will produce minimal or no length discrepancy or angular deviation and will seldom require treatment.
Growth disturbance resulting from a physeal fracture is usually evident 2–6 months after the injury, but it may take up to a year to manifest.9 This information is important both to warn the parents about possible complications and to anticipate close long-term follow-up. In fact the management of a posttraumatic growth disturbance is easier when directed exclusively towards treating the arrest rather than tackling both the arrest and the acquired deformity.
Growth disturbance is usually a consequence of the development of a bony bridge, or bar, across the physis. However, when the injury only reduces rather than stops the growth rate of a portion of the physis, it may still produce asymmetric growth and angular deformity with no development of bony bridge.74 If the bony bar involves a large portion of the physis, it may stop the physeal growth completely. However, in most cases the bony bar is confined to a rather small portion of the physis and the growth stops only at that point. The remaining healthy physis continues to grow, creating a tethering effect which may produce either a shortening or a progressive significant angular deformity, or both.
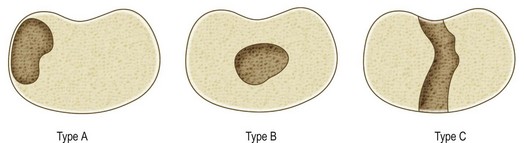
The extent and location of the bar, the skeletal age of the patient, and the amount of growth remaining from the physis must all be determined to plan the treatment of a physeal bar appropriately. Plain radiography, tomography, computed tomography (CT), or MRI44,75–78 may all be used to assess the anatomy of a physeal bar. MRI is by far the preferred method currently used to investigate physeal anatomy.79–82 In particular, fat-suppressed, three-dimensional, gradient-recalled echo sequences can provide an accurate three-dimensional reconstruction of the physis and estimate the percentage of physeal arrest.82 The classification of partial physeal arrests is based on their location within the physis (Fig. 30.7): peripheral (type A) or central (type B or C). In type B, the bar develops in the center of the physis and is surrounded by a perimeter of healthy physis. This may create a tethering effect that “tents” the epiphysis, leading to joint deformity. In type C (central) the bar traverses the entire physis (front to back or side to side) while the physis on both sides of the bar is normal.
Treatment/surgical technique
Treatment of physeal arrest
Completion of a partial physeal arrest and epiphysiodesis
Epiphysiodesis is an established method of limb length equalization or angular deformity correction in children and adolescents with projected limb length discrepancies at maturity as great as 5 or 6 cm. It consists of temporarily stopping or permanently destroying the activity of the whole or just a part (hemiepiphysiodesis) of the growth plate. First introduced by Phemister in 1933,83 epiphysiodesis has evolved into various reversible or irreversible, open or percutaneous techniques with and without instrumentation. The ideal tool should be minimally invasive, have minimal morbidity, and be reliably reversible. Pitfalls include errors in the prediction of growth and planning of the surgery. In particular with nonreversible techniques, timing must be precise. Overcompensation for limb length discrepancy or creation of an opposite angular deformity can be very distressing for the patient, family, and surgeon. Although many different techniques have proven their efficacy, percutaneous epiphysiodesis using transphyseal screws combines the minimal invasiveness of a percutaneous technique with reversibility.84
Physeal distraction
A distinction must be made between distractional epiphysiolysis and chondrodiastasis,85,86 chondrodiatasis,87 and hemichondrodiatasis.88
The chondrodiastasis technique employs large forces or rapid rate of distraction (>1 mm/day) or both.89 This provokes a distraction and opening of the physis that provides a rapid in situ correction from the bony bar without prior bar resection, but almost invariably it produces a premature physeal fusion.89,90 This notion limits the indications to patients nearing skeletal maturity and preventive lengthening according to predicted limb length discrepancy.91,92 In the literature, physeal distraction techniques are mostly reported for the treatment of lower-limb deformities and little is known about their application in the upper limb.
Bar resection
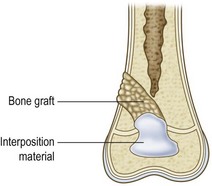
If a portion of the physis has prematurely closed, but the reminder of the physis is healthy and there is substantial growth remaining, resection of the physeal bar and insertion of interposition materials are indicated. This technique in fact preserves longitudinal bone growth ability.93,94 The procedure was first introduced by Langenskiold and has been documented in both human and animal models.45,76,93,95–98
The surgical technique of bar resection consists in removing the bone bridge along with the neighboring portions of metaphysis and epiphysis and filling the cavity with an inert material that will prevent recurrence of physeal bar formation (Figs 30.8 and 30.9).
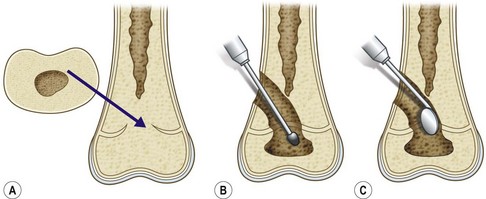
Fig. 30.8 (A) Central bar. (B) Bar resection through metaphyseal approach. (C) Assessing the resection.