Gram-Positive Infections Associated with Toxin Production: Introduction
|
Skin infections with the Gram-positive bacteria Staphylococcus aureus and group A streptococcus (Streptococcus pyogenes) are an important source of morbidity and even mortality. These bacteria produce toxins that can induce characteristic syndromes, including staphylococcal scalded-skin syndrome (SSSS) and toxic shock syndrome (TSS). Moreover, the production of these toxins is thought to underlie the ability of infection with these bacteria to initiate and/or to propagate inflammatory skin diseases.
Epidemiology
Factors in Toxin-Mediated Disease
Staphylococcal and streptococcal pathogenicity is due to the production of a range of immunomodulatory proteins including toxins, exoenzymes, and adhesins.1 Among the best characterized are the toxins (Table 177-1).
Bacteria | Toxin | Toxin Type | Clinical Disease |
|---|---|---|---|
Staphylococcus aureus | Exfoliatin type A | Epidermolytic | Bullous impetigo SSSS |
Exfoliatin type B | Epidermolytic | SSSS Bullous impetigo | |
TSS toxin 1 | Superantigen | TSS (menstrual > nonmenstrual), food poisoning | |
Staphylococcal enterotoxins A–C | Superantigen | TSS (nonmenstrual > menstrual), food poisoning | |
Streptococcus pyogenes | Streptococcal pyrogenic exotoxins A, C | Superantigen | TSS (nonmenstrual), scarlet fever |
Many risk factors play a role in the ability of a toxin-forming Staphylococcus or Streptococcus to produce disease. The development of disease is related to the resistance of the host to infection and to the virulence of the organism. Host resistance depends, among other factors, on intact skin and mucous membranes functioning as barriers to invasion and the host’s ability to mount an immune response (e.g., neutralizing antibodies) against such toxins. Colonization by virulent Staphylococcus or Streptococcus toxin-producing organisms exposed to optimal, focal conditions for growth and toxin production (e.g., menstruation + tampon use or abscess) allows these bacteria to initiate and/or propagate infection.
Minor defects in these barriers such as those produced by superficial excoriations, toe web fungal infections or alternatively, major defects produced by surgery, trauma, burns, or foreign substances (packing, sutures, intravascular catheters, shunts) increase the risk of infection. The role of host response cannot be overstated. For example, if a patient has an underlying immunodeficiency and cannot produce adequate neutralizing antibodies; or, as in chronic granulomatous disease where neutrophils lack the oxidative burst to destroy catalase-positive bacteria (e.g., S. aureus), the patient is at increased risk for colonization and infection by toxin-producing bacteria. Finally, the types of immune cells that are activated play an important role in the subsequent host response, especially as it relates to toxins that act as superantigens. The ability of a superantigen to activate numerous T cells is based upon the variable region of the T-cell receptor. This could result in various types of responses depending on the specificity of the individual T cell that happened to become activated. Table 177-2 provides an overview of Gram-positive coccal toxin related diseases.
| ||
Predominantly Cutaneous | Intermediate Cutaneous and Systemic | Predominantly Systemic |
|
|
|
Clinical Features | ||
|
|
|
Foci of Bacterial Colonization/Infection | ||
|
|
|
Diseases Caused by Exfoliative Toxins: Staphylococcal Scalded-Skin Syndrome
S. aureus-mediated production of exfoliative toxin also results in a spectrum of blistering skin disorders ranging from localized bullous impetigo (see Chapter 177) to a severe generalized form, SSSS. The syndrome is a generalized exanthematous disease consisting of cutaneous tenderness and widespread superficial blistering and denudation.
Exfoliative toxins (ETs) are made by certain strains of S. aureus (usually phage group 2). Exfoliatin A and B (ETA and ETB) are two serologically distinct proteins produced by S. aureus.2 ETs are serine proteases that bind to the cell adhesion molecule desmoglein 1 and cleave it, resulting in a loss of cell–cell adhesion.3 Consistent with the expression pattern of desmoglein 1, which is expressed in the upper part of the epidermis, the epidermolysis takes place usually between the stratum spinosum and granulosum. This results in a very thin-walled, flaccid blister that is easily disrupted, exhibiting a positive Nikolsky sign. The pathophysiology of ET resembles that of the autoimmune blistering disease pemphigus foliaceus, with desmoglein 1 targeted in both diseases. Presumably, staphylococcal bacteria have evolved this toxin to allow the bacteria to proliferate and spread beneath the barrier of the skin. There are two forms of ET-mediated disease: (1) localized bullous impetigo and (2) systemic SSSS. Recent studies suggest that the majority of cases of localized bullous impetigo are due to ETA, and systemic forms such as SSSS are due to ETB, possibly due to a lower titer of anti-ETB neutralizing antibodies in the general population.4 Although some reports suggest that ET can also act as superantigens (see Section “Diseases Caused by Superantigenic Toxins”) in addition to their epidermolytic activities,5 this has been called into question.6
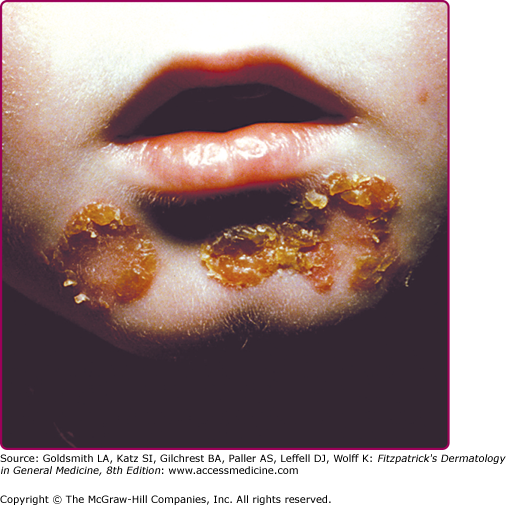
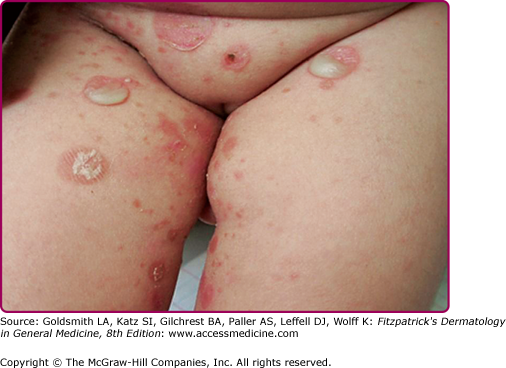
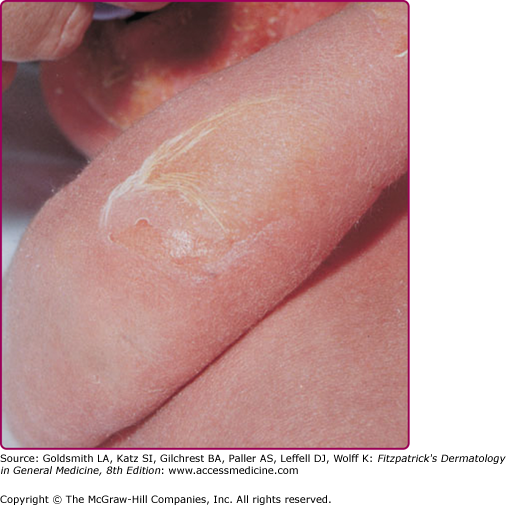
Infection of the upper part of the skin (epidermis) by S. aureus or S. pyogenes results in impetigo contagiosa (Figs. 177-1 and 177-2). Impetigo, which may account for up to 10% of all pediatric skin disease, consists of honey-colored crusts on an erythematous base. Approximately one-fourth of cases of impetigo are bullous, with local ET-expressing Staphylococcus and without hematogenous dissemination. As is the case with SSSS, bullous impetigo is usually a disease of children (see Chapter 177), although adult cases can also occur. The early lesions of bullous impetigo are cloudy vesicles or bullae surrounded by an erythematous rim (see Fig. 177-2). These blisters often rupture leaving superficial erosions. Lesions at various stages can often be seen. The lesions tend to be found around exposed parts of the body and around orifices (see Fig. 177-1). Diagnosis is usually based upon clinical appearance. In contrast to SSSS, confirmation of diagnosis can be easily obtained by aspiration of blister fluid for Gram stain and cultures will reveal S. aureus.
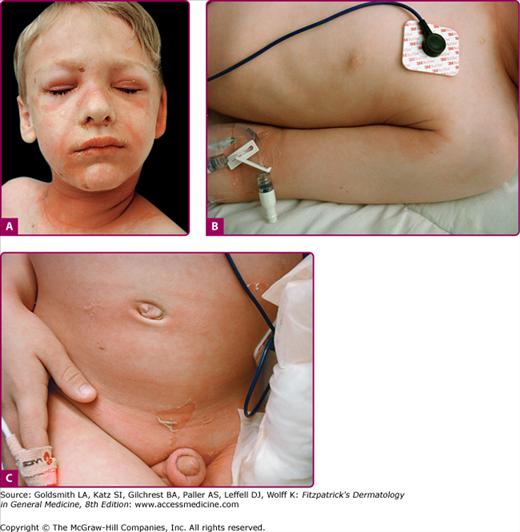
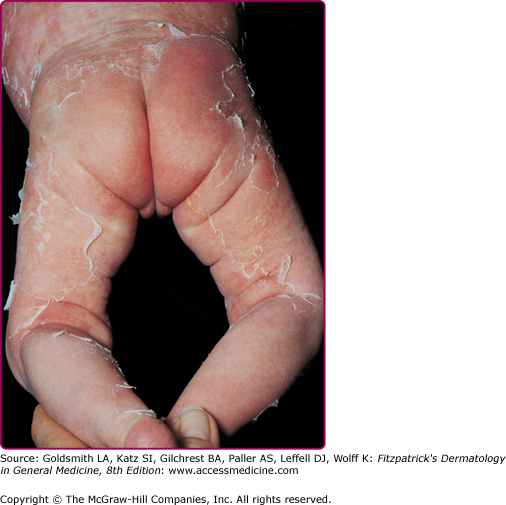
The clinical features of SSSS were first described in 1878 by the German physician Gotfried Ritter von Rittershain, who reported almost 300 cases of “dermatitis exfoliativa neonatorum” among young children.7 Outbreaks of SSSS tend to occur in clusters as a consequence of cross infection. Typically neonatal or maternity hospital staff colonized or infected with ET-producing Staphylococci are the source of these outbreaks. Although more commonly seen in infants/children, SSSS can also be seen in adults. The risk factors for adults include a compromised immune response allowing for growth of the S. aureus and possibly impaired amounts of toxin-neutralizing antibodies or renal insufficiency, which decreases the clearance of the toxin. Affected individuals initially have a faint, orange–red macular exanthem or uniform erythema (Fig. 177-3) sparing mucosal surfaces in association with a purulent conjunctivitis, otitis media, nasopharyngeal infection, or, occasionally, pyogenic skin infection such as bullous impetigo or that which arises from an umbilical stump or boil (carbuncle). These are the staphylococcal foci from which the toxin is released. Periorificial (see Fig. 177-3A) and flexural (see Fig. 177-3B) accentuation of the exanthema is often noted.
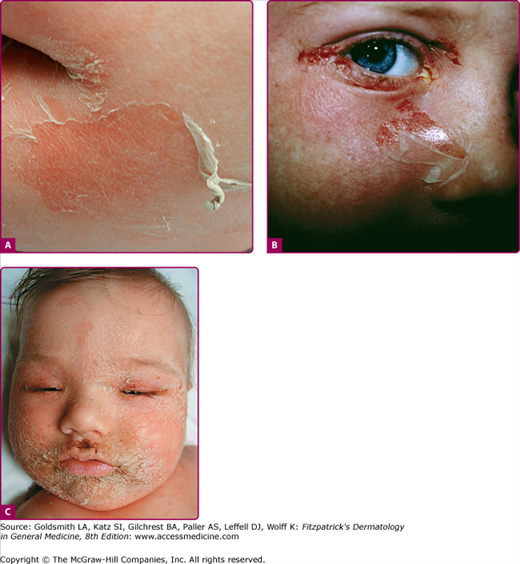
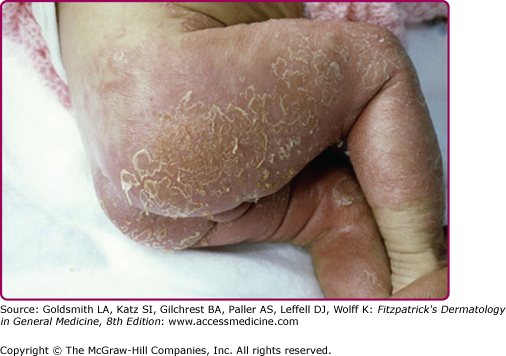
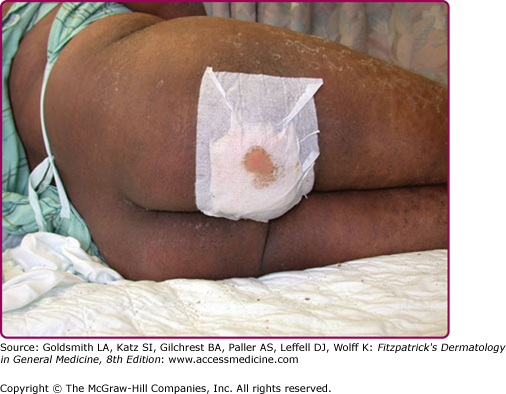
Although the early rash is not distinctive in appearance, the concomitant cutaneous tenderness is usually present at this early stage. Tenderness can often be so severe that infants will refuse to lie down or allow anyone to hold them. Within 1–2 days the rash progresses from an exanthematous scarlatiniform to a blistering eruption (Fig. 177-4; see Fig. 177-3C and eFig. 177-4.1). Very superficial tissue paper-like wrinkling of the epidermis, which is characteristic, progresses to large flaccid bullae in flexural and periorificial surfaces. A positive Nikolsky sign can be elicited by stroking the skin, which results in a superficial blister (see Fig. 177-4A and B and eFig. 177-4.1). Large sheets of the epidermal surface are typically shed, revealing a moist underlying erythematous base. At this stage, the disease looks very worrisome, resembling a generalized scalding burn. Although fevers are often present and outwardly the signs of SSSS may look serious, the infants and children do not usually appear toxic unless they have developed complications such as septicemia or pneumonia. The cutaneous process usually resolves spontaneously or faster with antibiotics and superficial desquamation, with healing completed within 5–7 days (Fig. 177-5; see eFig. 177-5.1). Cultures obtained from an intact blister are usually sterile, consistent with the pathogenesis of a hematogenously disseminated toxin originating from a distant focus of infection.
Figure 177-4
Staphylococcal scalded-skin syndrome (SSSS). Pictures of later SSSS demonstrating (A) the same patient in Fig. 177-3A 24 hours later with erythema, more superficial blisters with desquamation of large sheets. B. Superficial erosions around the eye with underlying denuded skin. C. Characteristic crusting with superficial erosions noted on face of this 10-month-old child with SSSS.
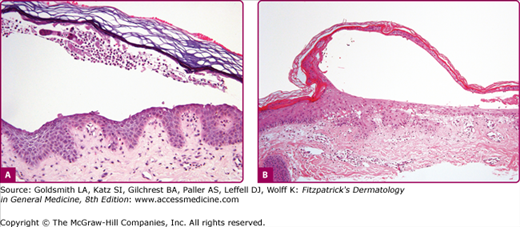
Although most cases of SSSS occur in infants and children, the syndrome can also rarely be seen in adults, particularly when there is renal failure (see eFig. 177-5.2). Histopathology shows acantholysis in the granular layer and subcorneal cleft formation in early lesions (Fig. 177-6B) and an intact viable epidermis with shedding of the stratum corneum in the desquamative stage.
Figure 177-6
Histology of bullous impetigo (200×) (A) vs. staphylococcal scalded-skin syndrome (100×) (B). In both conditions, the intraepidermal cleavage induced by the epidermolytic toxin occurs within or just below the stratum granulosum. Note paucity of cells in generalized disease (staphylococcal scalded-skin syndrome) (B) in comparison to the large numbers of leukocytes found in the localized form (bullous impetigo) (A). In both, cleavage occurs in the granular layer and is due to acantholysis. Note: free-floating acantholytic cells in A and compacted acantholytic cells in the blister roof of B.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree