Gram-Negative Coccal and Bacillary Infections: Introduction
The Gram stain is used to differentiate among different types of bacteria based on the biochemical properties of their cell walls.1 The stain is named after Danish scientist Hans Christian Gram (1853–1938) who developed the method to differentiate between two causes of pneumonia (Streptococcus pneumoniae and Klebsiella pneumoniae).2 The demonstration of Gram-negative cocci in sterile sites usually indicates infection due to Neisseria meningitides or N. gonorrhoeae. Gram-negative bacilli may be described as fusiform (e.g., Fusobacterium nucleatum, Capnocytophaga spp.), curved (e.g., Vibrio spp., Campylobacter spp., Helicobacter spp.), coccobacilli (e.g., Haemophilus spp., Brucella spp.), or rods (e.g., Escherichia coli, Klebsiella spp.).
Many of the characteristic cutaneous manifestations of infection with Gram-negative organisms are due to direct microbial invasion of the skin or subcutaneous tissues. In addition, responses of host cytokines, chemokines, receptor molecules (e.g., Toll-like receptors), and other effector cells and factors may contribute to fever, hypotension, and a variety of cutaneous manifestations.3–7
Infections Due to Neisseria Meningitidis (Meningococcus)
|
Three clinical syndromes associated with cutaneous involvement occur in meningococcal disease: (1) meningitis, (2) acute meningococcemia, and (3) chronic meningococcemia. Skin lesions are frequently the most dramatic manifestations of these infections. The presence of areas of purpura or gross hemorrhage is associated with higher numbers of organisms in the bloodstream and indicates a poorer prognosis than for a petechial eruption. The epidemiology, pathogenesis, and clinical presentations of these processes have been reviewed.3,5,6,8,9
Neisseria are aerobic, encapsulated, Gram-negative, bean-shaped cocci. Neisseria meningitidis grows well on blood-enriched media in 5%–10% CO2. With potentially mixed bacterial exudates, these organisms should be grown on a selective medium (e.g., modified Thayer-Martin). They can be distinguished from Neisseria gonorrhoeae by their fermentation of both glucose and maltose rather than of glucose alone. Meningococci are separable on the basis of capsular antigens, of which A, B, C, Y, and W-135 are the major human pathogenic groups. Outer membrane proteins that can identify serotypes are helpful in epidemiologic studies.5 In an epidemic of meningococcal disease, a shift of capsular group can occur (e.g., from group B to group C and vice versa), which facilitates evasion of host antibodies.10 Persons with late terminal complement deficiencies are especially prone to systemic infections with N. meningitides.11
Skin lesions associated with meningococcemia result from damage to small dermal blood vessels. Light and electron microscopy reveal bacteria within endothelial and polymorphonuclear cells in needle aspirate or punch biopsy specimens.12,13 Endothelial damage, thrombosis, and necrosis of the vessel walls occur. Immunoglobulins and complement are present, even in early lesions.5,12 Edema, infarction of overlying skin, and extravasation of red blood cells are responsible for the characteristic macular, papular, pustular, petechial, hemorrhagic, and bullous lesions. Many of the cutaneous hemorrhagic lesions may be caused directly by the effects of lipopolysaccharide (LPS) endotoxin or indirectly by stimulation of lease of outer membrane vesicles (blebs) has been observed on electron microscopy of meningococci in the plasma of patients with very high levels of circulating endotoxin in fatal meningococcal septicemia.14 Another contribution to the dermal inflammatory vascular injury, independent of immune complexes and cytokine activity, may be the interaction between vascular endothelium (intercellular adhesion molecule 1) and an adhesion glycoprotein (CD18) on leukocytes, the so-called dermal (localized) Shwartzman reaction (LSR).15 Mouse neutrophils express a surface protein, gp 49 B1, that prevents neutrophil-dependent vascular injury in response to LPS; humans have a leukocyte analog. The LSR requires a priming dose of LPS followed by a systemic challenge 18–24 hours later. A single intradermal injection of LPS into gp 49 B1-null mice produces an exaggerated microangiopathy in the LSR not seen in wild-type gp 49 B1 mice.16 LSR and the generalized Shwartzman reaction (in which both doses of LPS are administered systemically) serve as models for the thrombohemorrhagic vasculopathy, purpura fulminans, of meningococcal sepsis. The profound effects on small blood vessels, caused directly by bacterial invasion or indirectly by LPS, host cytokines, or LSR, may lead to diminished blood volume, lowered cardiac output, anoxia, myocardial failure, hypotension, acidosis, and diffuse intravascular coagulation (DIC).6 Purpura fulminans, the cutaneous manifestation of DIC (see Chapter 144), occurs in 10%–20% of children with meningococcal septic shock and is associated with functional impairment of protein C activation.8 The latter is thought to result from (1) downregulation of thrombomodulin and endothelial protein C receptor on endothelial surfaces to which protein C binds for its activation, (2) shedding of thrombomodulin from the endothelial surface, and (3) enzymatic cleavage of the thrombin-coupled protein C activation complex.17
Chronic meningococcemia is an uncommon disease, which usually begins as a nonspecific febrile illness over the course of weeks to months. It then establishes a pattern of recurrent fever, joint pain, and skin lesions that are present in over 90% of patients. Meningococci can usually be isolated from blood during the periodic fevers and rash. As with acute invasive meningococcal infection, chronic infection has been linked to absence of terminal complement components (see below).18,19 The chronic course of chronic meningococcemia, the lack of endotoxin-like manifestations, and the potential for eventual complications such as meningitis or endocarditis suggest that an unusual host-parasite relationship is central to this rare infection.
Humans are the only known natural hosts of N. meningitidis. Nasopharyngeal carrier rates vary with age: 1% in young children, 5% in those 14–17 years of age, and 20%–40% in adults. In the United States, there are 1,400–2,800 cases of meningococcal disease annually for a case rate of 0.5–1.1 in 105 total population.20 In schools and military camps, or when a carrier of a new strain develops disease in a day care or family setting, carrier rates can increase dramatically.21,22
Globally, group A strains have been responsible for epidemics of meningitis, especially in sub-Saharan Africa, where it accounts for about 80%–85% of all cases. In Europe and the Americas, groups B and C have predominated, and in the United States, a recent surge in the group Y disease has been reported. There is increasing evidence of serogroup W-135 being associated with outbreaks of considerable size. For example, in 2000 and 2001 several hundred pilgrims attending the Hajj in Saudi Arabia were infected with N. meningitides W-135.23
In most industrialized countries, a few clonal serogroup B electrophoretic types (e.g., ET-5 strains) have predominated during recent decades. Similarly, clonal complex ET-37 strains, usually expressing the serogroup C capsule but sometimes expressing serogroup B, W-135, or Y, are found around the world.5 Adult family members probably introduce the organism into the household, but secondary cases are most frequently spread from ill children to other family members in the same age group, especially under crowded conditions.24 Household spread is 500–800 times more frequent than the occurrence of secondary cases in the community, and there is an increased incidence of secondary cases in day care centers where large numbers of susceptible children congregate.6,20 Microbiologists who are exposed to droplets or aerosols containing N. meningitides (e.g., by spinning CSF) are also at higher risk of meningococcal disease.25 The absence of the spleen has been associated with fulminant meningococcal disease.20
Immunity to Meningococci increases with age, and protective bactericidal antibodies, both IgG and IgM, are found in 70%–95% of young adults. Newborns are often resistant to meningococcal disease until they are 3–6 months of age.26 Underlying immune defects that confer a predisposition to invasive meningococcal infection include functional or anatomic asplenia, a deficiency of properdin, and a deficiency of terminal complement components.6 Overall, 75%–85% of identified systemic bacterial infections occurring in individuals with complement deficiencies are cause by meningococci.11
The quadrivalent vaccine containing polysaccharides of groups A, C, Y, and W-135 had been the only licensed vaccine against meningococcal disease until 2005. Its inadequate protective efficacy in infants led to the development of protein polysaccharide conjugate vaccines that has high protective efficacy in children similar to the Haemophilus influenzae type b conjugate vaccine.27,28 Further, the vaccine has been associated with a reduction of carriage of the vaccine types included in the vaccine. The group B capsular polysaccharide, a polysialic acid polymer similar to fetal neural tissue, does not induce protective immunity. Efforts continue to develop a vaccine using outer membrane proteins of group B organisms and clinical trials are ongoing.29
The disease often follows a mild upper respiratory tract infection associated with headache, influenza-like complaints, nausea, and muscle soreness. These symptoms can be so short lived that fever, obtundation, and other manifestations of meningitis are the initial findings. In fulminant meningococcemia, vomiting, stupor, precipitous development of a hemorrhagic rash, and hypotension may be evident within a few hours of the onset of symptoms. Milder cases develop at a slower pace.
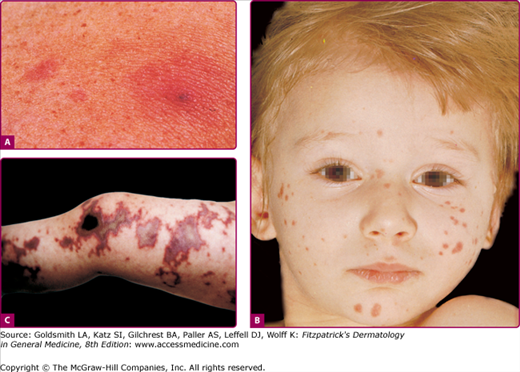
Skin findings in acute meningococcal infections are characteristically petechial, but transient macular or papular lesions (Fig. 180-1A), which can resemble those seen in viral exanthems, may be evident.30 The petechiae are small and irregular with a “smudged” appearance. Although most often located on the extremities and trunk, lesions can also be found on the head (see Fig. 180-1B), palms, soles, and mucous membranes. Extensive hemorrhagic lesions with central necrosis (suggillations) and bullae can develop. Gangrenous hemorrhagic areas (indistinguishable from purpura fulminans; see Chapter 144) can appear in severe meningococcemia, often with DIC (see Fig. 180-1C). Skin lesions and bacteremia are rarely seen with meningococcal pneumonia.31 Cellulitis has been noted occasionally, especially in children with meningococcal conjunctivitis (see Section “Primary Meningococcal Conjunctivitis”).
Figure 180-1
Neisseria meningitidis infection. Acute meningococcemia. A. Transient macular and papular lesions on the upper chest. B. Discrete pink-to-purple macules and papules, as well as purpura, on the face of a young child. These lesions represent early diffuse intravascular coagulation (DIC). C. Map-like gray-to-black areas of cutaneous infarction are seen in this child with DIC.
Patients with meningitis display signs of meningeal irritation and altered consciousness. Cranial nerve palsies, long-tract signs, seizures, and alterations in vital signs associated with changes in intracranial pressure may be present.
Obtundation and hypotension without meningeal signs, associated with the syndrome of DIC, are characteristic features of fulminant meningococcemia. Rarely, meningococcemia may result in septic foci in other areas: (1) septic arthritis, (2) purulent pericarditis with enlarging cardiac silhouette and findings of tamponade, and (3) bacterial endocarditis. More commonly, a delayed immune complex-mediated syndrome results in a sterile arthritis, pericarditis, or episcleritis.5 The acute arthritis–dermatitis syndrome, characterized by petechiae and nontraumatic arthritis, has traditionally been identified with disseminated N. gonorrhoeae infection (see Chapter 205). There has been a decline of gonococcal cases and an increase in cases of this syndrome caused by N. meningitidis.32
A polymorphonuclear leukocytosis is present in peripheral blood and cerebrospinal fluid (CSF) in meningitis. The CSF glucose value is commonly reduced. Characteristic organisms may be seen on Gram-stained smears of fluid, and Meningococci are usually isolated from the CSF. N. meningitidis may be isolated from the blood of approximately one-third of patients with meningitis and from almost 100% of patients with acute meningococcemia. Demonstration of organisms in cutaneous lesions has been variable, and the presence of Gram-negative commensal organisms on the skin requires cautious interpretation. Reports describe positive results for 50%–80% of aspirates, skin film samples, and punch biopsy specimens of petechial lesions.12,33
In patients already exposed to antimicrobial agents, the development of rapid, accurate, and inexpensive procedures for detection of soluble antigens in the CSF has been a major advance. The latex agglutination method and enzyme-linked immunosorbent assay methods are sensitive and very specific, but their clinical usefulness in most situations has been questioned.34 More recently, the polymerase chain reaction (PCR) has been used to detect N. meningitides in cerebrospinal fluid and/or blood.35 Further, PCR can be used to type strains, a useful adjunct in epidemic situations. However, PCR is not widely available.
Endothelial swelling is seen, a perivascular infiltrate of polymorphonuclear leukocytes is present, thrombi occlude capillaries and postcapillary venules, and the walls of capillaries and venules are destroyed, as in leukocytoclastic vasculitis (see Chapter 163).
Meningococcal infection warrants consideration in a patient with fever and a petechial or purpuric eruption, even in the absence of clinical meningitis (Box 180-1). Occasionally, the characteristic rash is absent, which delays consideration of meningococcal disease.
|
If untreated, acute meningococcal disease usually ends fatally. With treatment, recovery occurs in 90% of patients with meningitis.
In severe meningococcemia, especially with the rapid emergence of cutaneous hemorrhages, hypotension, and DIC, the entire course from onset to death can be measured in hours. In patients with sepsis-associated DIC, cutaneous thrombotic hemorrhages on the distal extremities may lead to skin and digital necroses that necessitate amputation.36 Rarely, young children with meningococcal sepsis, shock, and DIC suffer thrombotic injury that causes growth plate arrest.37 Myocardial dysfunction often occurs in meningococcal septic shock and is associated with high circulating levels of interleukin 6.38 These cases are often associated with massive adrenal hemorrhage (Waterhouse–Friderichsen syndrome). Children who die from meningococcal septic shock have relatively low cortisol levels (but not in the range expected in adrenal insufficiency).39 The mortality rate for meningococcal septic shock approaches 100%. Gradations in the severity of meningococcal disease make it difficult to assign an accurate prognosis, although a bedside predictive model has been proposed.6,40
The manifestations of chronic meningococcemia are vague at onset but tend to establish a pattern over a period of weeks or months.18,41,42 Initially, there may be an acute febrile illness, but it wanes and leaves the patient with intermittent muscle aches, joint soreness, mild headache, and anorexia with weight loss. The simultaneous emergence of a localized rash with several days of fever and joint soreness is characteristic. As fever recedes, the rash fades, and the patient may be totally free of skin manifestations for days or weeks. This pattern of recurring fever and rash may last from a few weeks to 6 or 8 months. Untreated cases may eventually evolve into acute meningococcemia, meningitis, or endocarditis. Several case reports have related this syndrome to the absence of a terminal component of complement, a finding also observed in sporadic and recurrent acute meningococcal infections.
Skin lesions are usually distributed about one or more painful joints or on pressure areas in contrast to the acral distribution in gonococcemia. They may vary in appearance and size (1–20 mm) from one crop of lesions to the next and include (1) pale to rose-colored macular and papular lesions (the most common type), (2) slightly indurated and tender erythema nodosum-like nodules, (3) petechiae of variable size, (4) petechiae with vesicular or pustular centers, (5) hemorrhage (minute) with an areola of paler erythema, and (6) grossly hemorrhagic areas with pale blue–gray centers.
Aside from the rash, the physical findings of chronic meningococcemia are minimal, except for occasional joint swelling and tenderness.
The skin lesions differ pathologically from those in acute meningococcemia in that bacteria are absent, and fluorescent antibody techniques do not detect meningococcal antigens. Also, thrombi do not occlude capillaries and venules, endothelial cell swelling is absent, and the perivascular infiltrate consists of mixed polymorphonuclear and mononuclear cells.
During febrile periods, blood cultures frequently reveal Meningococci and provide the specific diagnosis. Recently, a novel i–Neisseria menigitidis specific PCR assay was used to identify N. menigitidis in skin biopsy specimens even though blood cultures remained negative.43
A number of diseases with periodic fever, skin lesions, and joint involvement resemble chronic meningococcemia:
- Subacute bacterial endocarditis: a prolonged febrile course with a pleomorphic petechial rash, joint symptoms, and no overt focus make subacute bacterial endocarditis an important consideration. A prominent heart murmur, evidence of renal impairment, and blood cultures help establish the diagnosis.
- Acute rheumatic fever: when fever is prolonged, joint findings are prominent, and macular and papular rashes appear, the diagnosis may be acute rheumatic fever (see Chapter 160).
- Henoch–Schönlein purpura: the petechial hemorrhagic rash in Henoch–Schönlein purpura is more often symmetric, usually only on the lower extremities, and does not have the periodicity of the rash of chronic meningococcemia (see Chapter 163).
- Rat-bite fever, also referred to as Haverhill fever, may be acute (mimicking acute meningococcemia) or chronic (resembling chronic meningococcemia). Intermittent fever, rash, and joint manifestations are hallmarks of an illness that follows a rodent bite or ingestion of contaminated milk (see Chapter 183).
- Erythema multiforme: the iris-type configuration of lesions suggests erythema multiforme (see Chapter 39).
- Gonococcemia (chronic): the cutaneous and joint manifestations of chronic gonococcemia may continue for many days or weeks (see Chapter 205), and tenosynovitis in gonococcemia can be an important clue.
Some patients with chronic meningococcemia recover spontaneously without specific therapy, whereas others develop serious systemic complications, such as endocarditis or meningitis. The prognosis for treated infection is excellent.
N. meningitidis is responsible for up to 2% of cases of bacterial conjunctivitis. The source of infection is either direct inoculation of airborne organisms from close contact with carriers or manual contact with secretions from the patient’s own nasopharynx.44
Widespread emergence of sulfonamide-resistant strains, approximately 40 years ago, has complicated therapy for meningococcal infections. Initially, these strains belonged predominantly to serogroup B, but in recent years, they have been from serogroups A to C. More recently, increasing penicillin resistance has led to the recommendation to use a third-generation cephalosporin (ceftriaxone or cefotaxime) as primary therapy.45 Alternatives (provided the strain is susceptible) include penicillin G, ampicillin, fluoroquinolone, or aztreonam.45
The usual adult dosage of ceftriaxone is 2 g every 12 hours or cefotaxime 2–3 gm every 6 hours. Penicillin G (adult dose, 4 MU every 4 hours) or ampicillin (adult dose, 2 g every 4 hours) may be used if the penicillin MIC is less than 0.1 μg/mL. The usual duration of therapy is 7 days. Similar therapy should treat chronic meningococcemia. In highly penicillin-allergic adults, administration of chloramphenicol (1 g IV every 6 hours) is preferable to risking cross-reactions with a third-generation cephalosporin. However, increasing resistance of meningococcal strains to chloramphenicol worldwide could change this recommendation.46 All meningococcal isolates from blood, CSF, or other normally sterile body cavities should be tested for penicillin susceptibility.6 Immune complex-associated complications (fever, sterile arthritis, vasculitis, pericarditis, episcleritis) can occur in 15% of patients 4–10 days after initiation of antimicrobial therapy for severe meningococcal disease.47
Although it has been postulated that hypotension in acute meningococcemia may be due to adrenal failure associated with Waterhouse–Friderichsen syndrome (adrenal hemorrhage), blood cortisol levels and corticosteroid secretion rates have been found to be elevated or in the low normal range (not in the range associated with adrenal insufficiency).
The modern treatment of shock in sepsis begins with a number of well-accepted measures, including antibiotic selection, drainage procedures for abscesses, appropriate use of volume expanders, administration of β-adrenergic-stimulating drugs such as dopamine or isoproterenol, correction of severe acidosis, and, in selected patients, use of peripheral vasodilators. Therapies still considered experimental include those that neutralize host cell-mediated cytokines (e.g., interleukin 1, tumor necrosis factor) and protein C. Although numerous studies of glucocorticoid therapy have been completed, results have been variable, and on balance glucocorticoids do not appear to be indicated for septic shock.
Because of the impaired conversion of protein C to its activated form through the downregulation of thrombomodulin by inflammatory cytokines during sepsis, intravenous recombinant human activated protein C (drotrecogin alfa activated) has been evaluate for its efficacy as an adjunct in treating sepsis. However, a placebo-controlled randomized clinical trial in adults48 with severe sepsis and a low risk of death, and a placebo-controlled randomized trial in children49 with severe sepsis failed to demonstrate benefit. Neither of these trials included substantial numbers of patients with meningococcal sepsis. In an earlier randomized trial of patients with severe sepsis, recombinant human activated protein C reduced the mortality rate from 30.8% in the placebo group to 24.7% in those receiving drotrecogin alfa.50 The incidence of serious bleeding was 3.5% in the drotrecogin alfa group compared with 2.0% in the placebo group. Any patients with meningococcal infection with disseminated intravascular coagulation (DIC) should be closely monitored for increased bleeding. Severe meningococcal infections can be complicated by the syndrome of DIC. The basis for the diagnosis is usually a composite of associated hematologic abnormalities, including thrombopenia and hypofibrinogenemia, prolongation of the prothrombin time and partial thromboplastin time, and the presence of fibrin split products. If bleeding occurs, it may be necessary to administer fresh frozen plasma. Treatment for each case must be individualized, because therapy may be harmful and produce more problems than the DIC syndrome. Data on the use of heparin in treating DIC remain unconvincing, and because of potential adverse effects, this drug is no longer recommended. Investigators continue to study antibodies to various cytokines whose levels are elevated in meningococcal septicemia and immunomodulatory therapies.51
There is a critical need for reliable chemoprophylaxis to rapidly protect children exposed in a day care nursery, contacts in a crowded household, and institutional groups, because secondary cases can emerge, often within 24–48 hours of an index case.21,52
Rifampin, ceftriaxone, ciprofloxacin, and azithromycin are appropriate drugs for chemoprophylaxis in adults. The drug of choice for most children is rifampin (>1 month of age, 10 mg/kg, maximum 600 mg, orally, every 12 hours for 2 days).53 However, rifampin resistance occurs and reports have documented the development of invasive meningococcal disease. Fortunately, ciprofloxacin and ofloxacin are effective single-dose substitutes, and ceftriaxone is available for parenteral single-dose use in children as well as adults. Azithromycin has demonstrated the ability to clear carriage in one study.
The meningococcal conjugate vaccine (MCV4 which contains groups A, C, Y, and W-135) is preferred for children, adolescents, and adults less than 55 years of age. MCV 4 is indicated for children 2 through 10 years of age with persistent complement component deficiency, anatomic or functional asplenia, and certain other conditions placing them at high risk. It is also indicated for all adolescents at 11 or 12 years of age (catch period, 13 through 18 years of age). MCV4 is indicated for adults less than 55 years of age who have the following risks: anatomic or functional asplenia, persistent complement component deficiencies, first year college students living in dormitories, microbiologists routinely exposed to isolates of N. meningitides, military recruits, and persons who travel to or live in countries in which meningococcal disease is hyperendemic or epidemic.
The polysaccharide vaccine (groups A, C, Y, and W-135 N. meningitidis) is safe and effective in preventing meningococcal disease in adults and is preferred for adults who are 56 years of age or older. Indications are the same for adults of 55 years of age or younger.
Elucidation of the complete genome of N. meningitidis has identified several conserved proteins that, in an experimental mouse model, have been protective against challenge with all pathogenic N. meningitidis organisms.
Patients, who present with cutaneous findings that are consistent with acute meningococcal infection, represent a medical emergency. Because the course of the disease is so rapid, it is imperative that appropriate diagnostic samples (i.e., blood and cerebrospinal fluid) be collected in an expedient manner and then the patient started immediately on empiric antibiotic therapy. If cerebrospinal fluid cannot be rapidly obtained, then the patient should be started on empiric therapy and the cerebrospinal fluid obtained as soon as feasible. All patients with known or suspected invasive meningococcal infection should be placed on Droplet Precautions (private room, healthcare providers wear a surgical mask when in the room). If the patient is in an open area (e.g., emergency department), a surgical mask should be placed over the patient’s mouth and nose (if possible). Droplet precautions should be continued until the patient has been on appropriate therapy for at least 24 hours.
Infections Due to Neisseria Gonorrhoeae (Gonococcus)
Neisseria gonorrhoeae are nonmotile, nonspore forming, Gram-negative cocci that typically grow in pairs (i.e., diplococci). They closely resemble Neisseria meningitides in appearance. Traditionally, Gonococci are differentiated from other Neisseria species by their ability to grow on selective media. Gonococci are intolerant of drying, and therefore patient samples should be immediately inoculated onto the appropriate culture medium.
Neisseria gonorrhoeae primary infects columnar or cuboidal epithelium. Attachment to mucosal epithelium is mediated in part of pili and Opa (opacity related proteins or protein II). Infection is characterized by four stages: (1) attachment to the mucosal cell surface; (2) local penetration to the submucosal tissues; (3) local proliferation; and (4) a local inflammatory response or dissemination.
Generally, human serum will kill circulating Gonococci via activation of the complements system. However, strains causing disseminated gonococcal infection (DGI) are typically serum resistant and induce defective deposition of complement on the outer membranes.54 Gonococci have developed multiple mechanisms for evading host defenses including antigenic and phase variation of pili and Opa, masking of gonocococcal antigen, release of IgA1 proteases, and production of blocking antigen.
Gonorrhea is the second most commonly reported communicable disease in the United States with more than 300,000 cases reported annually. From the mid-1970s through the mid-1990s, the incidence of gonorrhea declined approximately 75%. However, in the past 15 years, the incidence appears to have stabilized at around 110–120 cases per 100,000 population. The incidence of gonorrhea is lower in Western European countries.
The highest rates of gonococcal infection are reported in adolescents and young adults, minorities, and persons living in the Southeastern United States. Traditionally, higher rates have been reported in men as compared to women. However, most recently nearly equal rates have been reported in men and women. Risk factors for infection include a new sexual partner or multiple sexual partners, younger age, minority ethnicity, low education and socioeconomic levels, substance abuse, unmarried status, and previous infection. The attack rate after sexual exposure has often been documented to be 50% or greater.
Over the years, N. gonorrhoeae has demonstrated the ability to develop resistance to clinically important antibiotic therapies. In the 1980s, N. gonorrhoeae developed resistance to penicillin and tetracycline. More recently, fluoroquinolone resistance has been reported across the Unites States with prevalence up to 20% in parts of California and Hawaii.
Gonococcal infections lead to different clinical syndromes in men and women. In men genital infections are generally symptomatic. Most commonly infection leads to urethritis, but may occasionally progress to periurethral abscess or acute prostatitis. Infection may involve the pharynx or rectum, especially in men who have sex with men. Disseminated infection is less common in men compared to women.
Urethritis is characterized by penile discharge and dysuria. The discharge may be purulent or mucopurulent in color and copious. Microscopic analysis of the urethral secretions reveals white blood cells. Epididymitis characterized by testicular pain and swelling, which is usually unilateral, may occur. Epididymitis must be distinguished from other causes of testicular pain such as torsion or trauma.
In women, as in men, gonorrhea can involve any portion of the genital tract. Women are more likely than men to have asymptomatic infection. The most common site of mucosal infection in women is the cervix. Symptomatic infection is characterized by vaginal pruritis and/or a mucopurulent discharge. Pain is uncommon. Women may also develop urethritis, anorectal infection, or proctitis. Pharyngeal infection may occur and is usually asymptomatic. Cervical gonorrhea leads to pelvic inflammatory disease in 10%–40% of women. Even in the absence of symptoms, substantial scarring and inflammation may occur resulting in decreased fertility. Symptoms of pelvic inflammatory disease include pelvic and abdominal pain, vaginal bleeding, and dyspareunia.
Disseminated gonococcal infection (DGI) occurs in 1%–3% of persons infected with N. gonorrhoeae. Most patients with DGI are less than 40 years of age. Both males and females may be affected, although DGI is approximately three times more common in women than men. Risk factors for DGI include recent symptomatic genital infection in both men and women, recent menstruation, pregnancy, congenital or acquired complement deficiencies (C5, C6, C7, or C8), and systemic lupus erythematosus.
DGI typically present with one of two syndromes.55–60 First, a triad of tenosynovitis, dermatitis, and polyarthralgias without purulent arthritis. Second, purulent arthritis without associated skin lesions. Frequently there is overlap between these two syndromes. In the tenosynovitis, dermatitis, polyarthralgias syndrome, the acute phase is characterized by fever, chills, and malaise. Tenosynovitis is classic. Skin lesions are the most common manifestation of DBI and occur in 50%–70% of patients. The eruption typically appears during the first-day symptoms and may recur with each episode of fever. The skin lesions associated with DGI begin as tiny red papules or petechiae 1–5 mm in diameter, many of which evolve rapidly through vesicular or pustular stages to develop a gray, necrotic center, often on a hemorrhagic base. Papules, bullae, pustules, and hemorrhagic lesions, all may be present simultaneously. The lesions tend to be scanty but widely distributed. The distal portions of the extremities are most commonly involved with sparing of scalp, face, trunk, and oral mucous membranes. Histologic exam reveals leukocytoclastic vasculitis with fibrin thrombi. Circulating immune complexes may play a role in the pathogenesis of DGI-associated skin lesions and arthritis/tenosynovitis.
Uncomplicated urogenital and anorectal infection is best diagnosed using nucleic acid amplification. In men, this may be performed on urethral discharge or urine and in women on cervical, vaginal, or urine samples. Culture remains the gold standard with samples immediately plated on Thayer–Martin medium. While culture is 100 specific, sensitivity is only between 65% and 85% in women. Urogenital and anorectal infection should be treated with a single injection of ceftriaxone.61 Alternatively, cefixime may be used for oral therapy. In patients who have a severe allergy to β-lactam antibiotics and cannot undergo desensitization, azithromycin should be used. However, more than 5% of recent strains have demonstrated decreased susceptibility to this drug. Spectinomycin is another alternative therapy in the β-lactam allergic patient.
In patients who present with DGI, it is best established via synovial fluid analysis that generally reveals around 50,000 cells/mm3. Patients with tenosynovitis, dermatitis, and polyarthralgia typically have negative synovial fluid cultures. All patients with suspected DGI should have two sets of blood cultures prior to therapy. Although blood cultures are frequently negative, when positive they confirm the diagnosis. Patients should also have synovial, skin, urethral or cervical cultures, and rectal cultures immediately plated on appropriate media. Approximately, 50% of patients will have a positive mucosal culture. More recently, molecular diagnostics have been used to diagnose culture-negative DGI include real-time PCR of synovial fluid62 and nucleic acid amplification of a skin biopsy sample.63 A variety of syndromes may mimic DGI including acute hepatitis B, bacterial arthritis, acute rheumatic fever, infective endocarditis, Reiter’ syndrome, HIV seroconversion syndrome, rheumatoid arthritis, and psoriatic arthritis. DGI may be treated with intravenous ceftriaxone or cefotaxime with the treatment course being completed with oral cefixime. All patients with DGI should be evaluated for Chlamydial infection and HIV. Whenever possible, sexual partners should be identified and offered treatment. Patients with recurrent DGI should be screened for deficiencies of the terminal components of complements.
Infections Due to Pseudomonas Aeruginosa
|
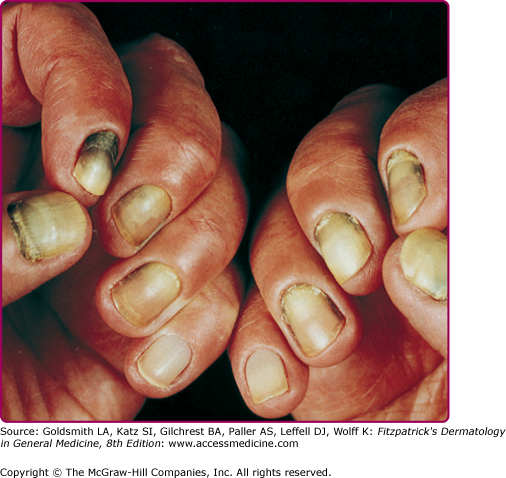
Pseudomonas aeruginosa is a nonfermentative, obligately aerobic, Gram-negative bacillus. Some strains produce a blue pigment (pyocyanin), a water-soluble yellow–green substance (fluorescein), or black pyomelanin. With Wood’s lamp, the presence of organisms in lesions of skin or nails can be detected if the infecting strain produces fluorescein. The pigments impart a characteristic greenish color to the surrounding growth media or the tissue substrate involved in clinical disease (e.g., “green nail” syndrome, Fig. 180-2). In addition, an odor of grapes, characteristic of trimethylamine, often accompanies the growth of these microorganisms.
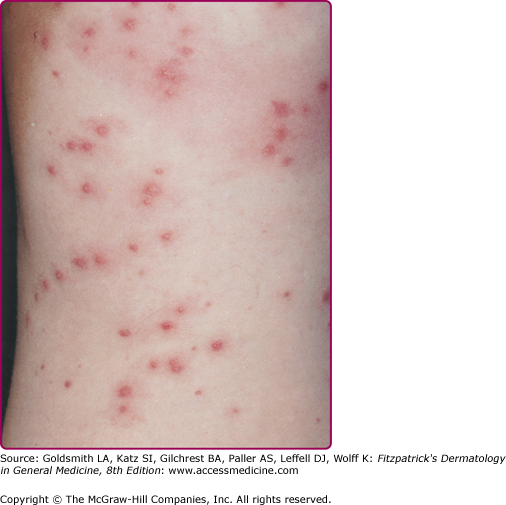
Organisms gain entry at sites of maceration, dermatophytic foci, trauma, or foreign bodies (e.g., indwelling venous catheters), or via aspiration into the respiratory tract. Infections in otherwise healthy individuals are unusual; when they occur, the involved regions are often areas with increased moisture (e.g., toe webs, the external auditory canal). Infection may begin in the base of the nail in persons who frequently have their hands or feet in water. This can progress to paronychia, followed by development of a green–blue discoloration of the nail due to local pigment production. The ability of this organism to infect healthy, but moistened, skin is evidenced by the occurrence of disseminated papules and pustules on areas of skin of people immersed in hot tubs (“hot tub folliculitis,” Fig. 180-3), wading pools, and swimming pools.52 These ubiquitous organisms can sometimes be aggressive secondary invaders in open wounds, in decubitus and other skin ulcerations, or in association with thermal burns. Rarely, a superficial pyoderma due solely to Pseudomonas is engrafted on a generalized or localized dermatitis, such as tinea pedis or eczema, producing irregular pustular areas with macerated borders.64 Serious invasive infections occur in debilitated patients; malnourished infants; individuals whose normal bacterial flora has been suppressed by antibiotics; patients with neoplastic diseases, granulocytopenias, or impaired circulating or cellular immunity, including that associated with acquired immunodeficiency syndrome (AIDS)65; and individuals requiring mechanical respiratory assistance. These organisms frequently colonize body surfaces and survive exposure to antibiotics. P. aeruginosa can spread widely via the bloodstream, producing a disseminated infective vasculitis in which organisms can appear in the adventitia and media of vessels without luminal clots. Occasionally, features of the generalized Shwartzman reaction are found, but less frequently than in infections due to N. meningitidis or Enterobacteriaceae.
Because P. aeruginosa is widely distributed in nature, it is not surprising that it may contaminate plants, vegetables, hot tubs, and swimming and wading pools. Moist regions of skinfolds and the external auditory canal are the most common sites of colonization (3%–5% of cases). P. aeruginosa is found in small numbers in the feces of 10%–20% of the population.
Any activity that leads to excessive local moisture—laundry work, dishwashing, or hiking for long periods in wet terrain—will enhance the growth of these organisms. Moist or weeping cutaneous lesions (e.g., thermal burns) encourage the growth of Pseudomonas as well as of other Gram-negative bacteria. Increased environmental humidity is frequently associated with overgrowth of these organisms. Synthetic sponges easily become contaminated with Gram-negative bacteria and have served as vehicles of transmission.
Systemic infection with P. aeruginosa depends primarily on altered susceptibility of the host rather than on spread from individual to individual or on increased pathogenicity. However, exceptions may be seen in newborn nurseries, respiratory care units, and occasionally urology wards, where dissemination from a primary source may occur. Neutropenic or immunocompromised hospitalized patients are more likely to become colonized and develop invasive disease. P. aeruginosa is the sixth most common pathogen in healthcare-associated infections (ranks seventh for central line-associated bloodstream infections, fourth for catheter-associated urinary tract infections, second for ventilator-associated pneumonia, and fifth for surgical site infections).66
Pseudomonas produces a number of characteristic lesions. In addition, colonization by these organisms complicates other skin diseases and open wounds.51
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree