Gracilis Flaps for Breast Reconstruction
Karen M. Horton
Introduction
The inner thigh skin and fat based on the transverse upper gracilis (TUG) musculocutaneous flap blood supply provides an autologous donor area with several qualities complementary to microvascular breast reconstruction. The gracilis flap is an exciting alternative to abdominal, back, or buttock tissue flaps for reconstruction of a natural-looking and soft, shapely breast.
The gracilis muscle has a consistent and reliable blood supply and has been well described (1,2). The TUG flap can be shaped to mimic a mastectomy specimen, providing excellent contour and projection to the breast reconstruction. The characteristics and skin color of the TUG flap allow for immediate nipple-areola reconstruction in both immediate reconstruction following skin-sparing mastectomy and in delayed breast reconstruction. TUG flap microvascular breast reconstruction is an excellent option for patients who desire autologous reconstruction and who do not have adequate abdominal donor tissue or do not desire abdominal scars.
History
Use of the TUG myocutaneous flap for breast reconstruction was first described as a single breast reconstructive case in 1992 (3). The cutaneous territory of the gracilis myocutaneous flap was demonstrated by anatomic and injection studies to lie perpendicular to the muscle in its proximal one third, transverse and parallel to the medial groin crease. Based on the direction of exit of cutaneous perforators in the superomedial thigh region, the transverse cutaneous skin paddle of the gracilis muscle has since been accepted as dominant, much like the lower transverse paddle of the rectus abdominis muscle (3). Perforators extending through the gracilis muscle vascularize the area, reaching from over the adductor magnus and sartorius muscle anteriorly to the midline of the thigh posteriorly (4).
Although the vertical paddle of the gracilis has been used for breast reconstruction (5), it is accepted as much less reliable (6) and has a more visible vertical scar. We now offer inner thigh free flap reconstruction using the transverse skin paddle to patients without adequate abdominal donor tissue and to those patients who do not wish to have postoperative scars associated with abdominal tissue harvest.
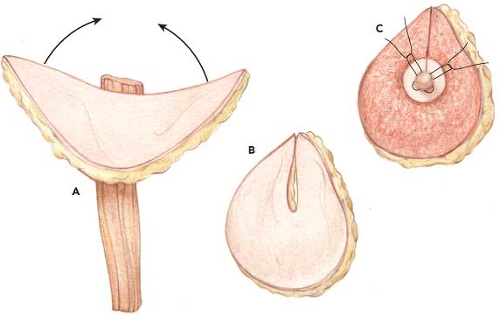
The transverse upper gracilis flap technique is relatively straightforward and reliable and can be aesthetically superior to abdominal reconstruction in two significant ways: (a) it has the advantage of allowing for immediate nipple-areolar reconstruction, negating the need for secondary surgery, and (b) coning of the flap into a projecting breast shape is simpler than for abdominal flaps. On the basis of a semilunar construction of the skin paddle, this flap provides excellent dimensions and good projection and can be contoured for immediate nipple-areola reconstruction. The aesthetics of this type of reconstruction can be excellent.
Transverse Upper Gracilis Flap Design
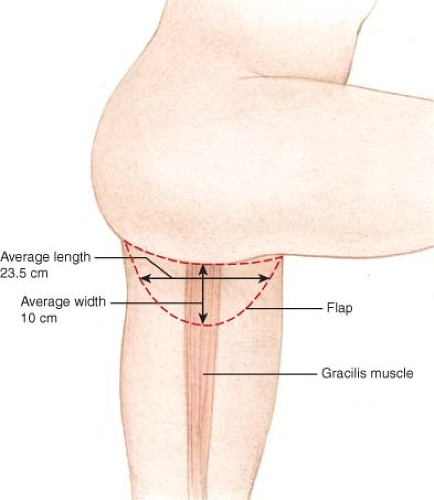
The TUG flap is designed with a semilunar skin paddle transverse to the longitudinal axis of the gracilis muscle in the inner thigh (Fig. 69.1) The superior aspect of the flap is marked approximately 1 cm below the groin crease anteriorly and centrally but extends well into the gluteal crease at the most posterior aspect. Placement of the incision slightly below the crease avoids distortion of the labia majora with related symptoms, as can occur in medial thigh lift (7).
The anteroposterior length of the flap extends up to 28 cm. The width of the flap is judged by pinching the inner thigh tissue with the thighs in adduction, using the maximum width that can be easily closed without tension. The flap has been designed as wide as 11 cm at the central axis over the gracilis muscle (Fig. 69.2). A pencil Doppler probe is used to confirm the location of perforating vessel(s) over the gracilis muscle and into the skin paddle (Fig. 69.3).
The procedure is performed with the patient in the supine position, with the thigh abducted and the knee flexed. The flap is harvested with the patient in well-padded obstetric-gynecologic operative stirrups, which facilitates dissection and closure of the posterior aspect of the wound.
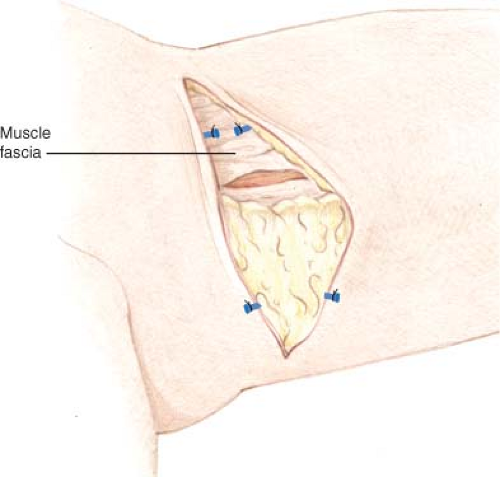
The anterior thigh incisions are made first. The posterior branch of the saphenous vein is harvested with the flap (Fig. 69.4), and any anterior venous branches are left in situ, although they can be included in the skin paddle if needed. Lymph nodes are avoided and are left in situ to avoid the risk of lower extremity lymphedema.
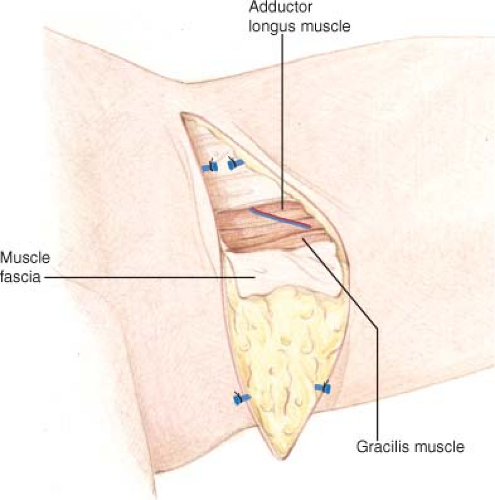
Anterior flap dissection proceeds superficial to the muscular fascia until the medial/posterior edge of the adductor longus is encountered (Fig. 69.5). Beveling of subcutaneous adipose tissue is used to maximize the bulk taken with the flap. The deep fascia is incised longitudinally, and the space between the adductor longus and gracilis muscle is separated and the vascular pedicle to the gracilis is identified. Pedicle dissection proceeds proximally to the origin from the superficial femoral artery. Posterior dissection then continues superficial to the muscular fascia, entering the deep fascia at the posterior aspect of the gracilis. Pedicle length ranges from 6 to 8 cm.
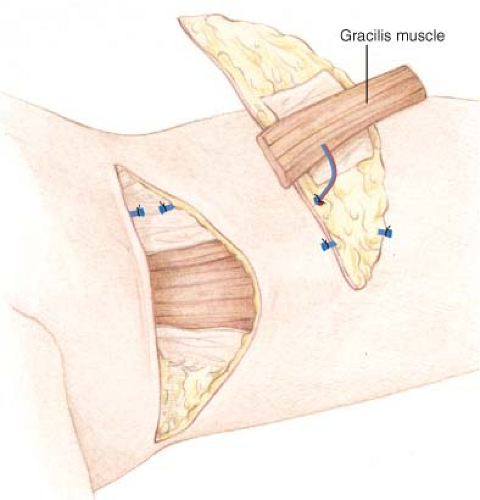
The gracilis muscle is transected superiorly and inferiorly, commonly taking only a portion of muscle lying directly beneath the flap (Fig. 69.6). Additional inferior muscle length may be optionally included for enhanced flap volume. Usual flap elevation time is approximately 45 minutes or less.
Following pedicle division, absorbable sutures are used to maintain flap coning and achieve projection (Figs. 69.7 and 69.8). The gracilis muscle may additionally be used to increase
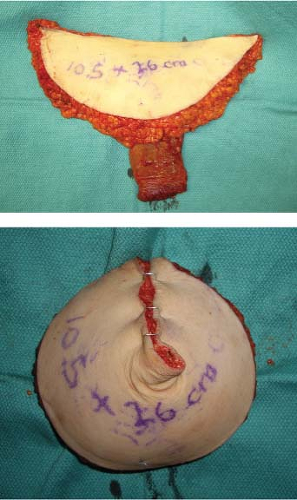
projection by securing it posteriorly behind the adipose tissue, with predictable postoperative muscle atrophy. In immediate reconstructions, the mastectomy specimen is weighed and measured for comparison with the TUG flap, typically an appropriate match in terms of volume and dimensions. Flap projection has often been greater than the native mastectomy specimen (Fig. 69.9).
projection by securing it posteriorly behind the adipose tissue, with predictable postoperative muscle atrophy. In immediate reconstructions, the mastectomy specimen is weighed and measured for comparison with the TUG flap, typically an appropriate match in terms of volume and dimensions. Flap projection has often been greater than the native mastectomy specimen (Fig. 69.9).
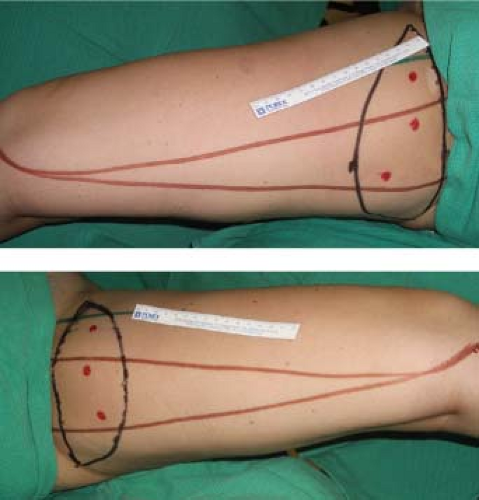 Figure 69.2. Intraoperative markings for transverse upper gracilis flap. Note anterior and posterior borders of the gracilis muscle and pencil Doppler markings of perforator signals. |
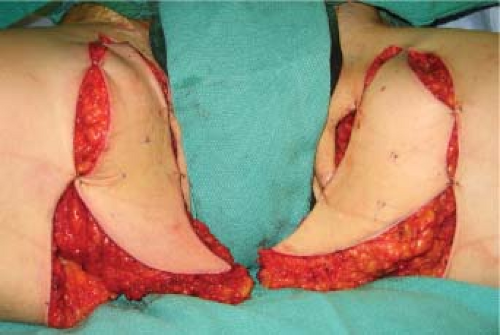 Figure 69.3. Elevation of transverse upper gracilis flaps in situ. Prolene suture marks the location of Doppler signals. |
The flap is deepithelialized, except for an areolar circle in immediate reconstructions (Figs. 69.9D and 69.10), and is completely deepithelialized in nipple-sparing mastectomy. An areolar circle is created and accentuated by a circumareolar incision for delayed reconstructions, deepithelializing and burying of the superior flap beneath the native mastectomy skin (Fig. 69.11).
Microvascular anastomosis is usually to the internal mammary system beneath the third or fourth costal cartilage. Following coning, the pedicle enters the undersurface at the center of the flap, enabling inset of the flap in any orientation desired (Fig. 69.12). Postoperative flap assessment includes clinical and external pencil Doppler monitoring if the flap is exposed, in addition to continuous implantable venous Doppler probe monitoring.
The inner thigh donor area is closed with interrupted sutures in the deep fascia (7), interrupted deep dermal and continuous subcuticular skin sutures over a suction drain exiting from the superior aspect of the thigh wound.
Immediate nipple-areolar reconstruction is performed by folding the semilunar flap and accentuating the apex of the resultant standing cone as the area of maximum projection using interrupted horizontal mattress sutures (Fig. 69.10). Care is taken not to create excessive suture tension to avoid circulatory compromise to the nipple reconstruction. An areola circle is drawn, and skin surrounding this circle is deepithelialized and buried beneath the mastectomy flaps prior to microvascular anastomosis. The naturally darker pigment of the inner thigh defines the areolar reconstruction.
Patients are placed on postoperative aspirin as an anticoagulant for 1 month and allowed to ambulate at 2 to 3 days postoperatively. Hospital stay averages 5 days.
Results
A total of 48 flaps in 28 patients were performed from 2005 to June 2009. Patient age ranged from 31 to 64 years (average age 49 years). All patients had a history of breast cancer or high personal risk of breast cancer (strong family history or BRCA gene positivity). Median follow-up was greater than 1.5 years (range 3 months to 4 years). Nine breasts were reconstructed in the face of previous radiation. Twenty-seven flaps were reconstructed immediately following mastectomy, and 21 were delayed reconstructions (Table 69.1).
One patient had a previous abdominoplasty and failed implant reconstruction. Twenty-three patients had inadequate abdominal donor tissue; three patients had previous deep inferior epigastric artery perforator (DIEP) or transverse rectus abdominus musculocutaneous (TRAM) flap breast reconstruction. Three patients elected to undergo TUG flap reconstruction in lieu of abdominal tissue reconstruction in order to avoid the abdominal donor site scars.
Flap weight has ranged from 238 to 648 g (mean 367 g). Flap length averaged 23.5 cm (range 20 to 28 cm), and flap width averaged 10 cm (range 8 to 12 cm). Microvascular success was 98%, with a single flap failure from combined venous and arterial thrombosis in the face of preoperative high-dose chest wall radiation. A second patient was taken back to the operating room 12 hours after reconstruction because of venous thrombosis. This flap was salvaged by thrombectomy and vessel repair, and the flap survived with zero flap loss or fat necrosis.
There was one case of poor venous outflow of the skin paddle, in spite of adequate muscle outflow as measured by pencil and venous Doppler. This flap demonstrated prolonged edema with complete softening of the flap over 3 months and no resultant fat necrosis.
Forty flaps included immediate nipple-areolar reconstruction (83%), with 23 immediate and 17 delayed cases. Two flaps were completely buried beneath nipple-sparing mastectomies, with the standing cone of the flap placed beneath the native nipple of the mastectomy for additional projection.
Eight donor sites developed partial dehiscence of donor areas at the greatest point of tension (Table 69.1). Four were treated with local dressing changes and healed by secondary intent, and four were debrided and closed over a suction drain under general anesthetic. One patient with bilateral donor-site breakdown had an asymptomatic atypical urinary tract infection that contributed to wound dehiscence and poor healing; debridement and vacuum-assisted closure and antibiotic therapy enabled healing by secondary intent. Four flap donor sites developed postoperative seromas, of which two required operative drainage and seroma capsule excision. The other two seromas were treated with regular needle aspiration and resolved with compression and time. There has been no functional loss at the donor area, and all patients have resumed
normal activity, including horseback riding, running, cycling, and professional dance instruction.
normal activity, including horseback riding, running, cycling, and professional dance instruction.
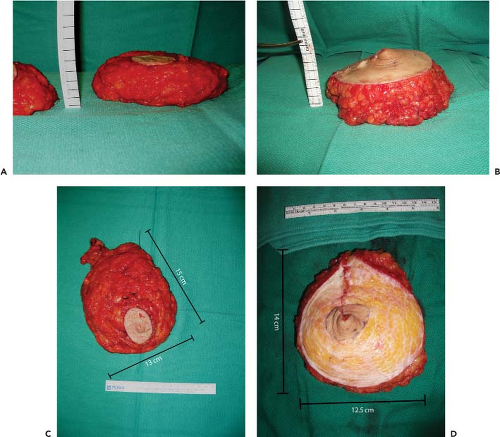 Figure 69.9. Flap projection of the mastectomy specimen (A) and of the folded transverse upper gracilis (TUG) flap in the same patient (B). Markings are added to the rulers for clarity, revealing 1-cm ruler lines. Flap projection is greater than the mastectomy specimen by approximately 1 cm. The mastectomy specimen (C) is similar in size, shape, and weight to the deepithelialized flap (D). Mastectomy specimen weight was 444 g, and the inner thigh flap was 426 g. The contralateral specimen was 496 g, and the TUG flap was 488 g.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|