Free Transverse Rectus Abdominis Myocutaneous Flap Breast Reconstruction
Chet L. Nastala
Minas T. Chrysopoulo
Steven M. Pisano
Peter R. Ledoux
Introduction and Histor
Most practices proficient in the techniques of autologous tissue transfer consider the free transverse rectus abdominis myocutaneous (TRAM) flap and, more recently, the deep inferior epigastric perforator (DIEP) flap the gold standards for breast reconstruction. These microsurgical tissue methods are preferred because they offer superior aesthetic results; a soft, warm, natural-feeling reconstructed breast; the ability to achieve optimal symmetry with the opposite native breast; a potentially sensate reconstructed breast, with the addition of a microneurorrhaphy; and a “durable” result that is maintained, or even improves, over time. Superior vascular perfusion and the avoidance of an upper epigastric bulge secondary to muscle tunneling are additional benefits over the pedicled TRAM flap.
The microvascular free TRAM and DIEP flaps offer flexibility in breast reconstruction. These techniques can be used for primary and secondary reconstructions (to salvage a failed implant or other non-TRAM or non-DIEP flap procedure), in cases in which the chest wall has been irradiated, and in circumstances in which the residual native breast skin–chest wall envelope is exceedingly thin (Fig. 55.1) or absent following extensive tissue resection (Figs. 55.2 and 55.3). Use of the free TRAM or DIEP flap allows one to avoid the pitfalls of tissue expander implant reconstructions: infection, malposition, contracture, deflation, and infection (1).
Implant reconstruction patients often require multiple procedures aimed at maintaining the breast mound. Furthermore, long-term resource costs associated with a tissue expander/implant reconstruction may be higher, depending on practice location and surgeon experience and preference, than those associated with a free TRAM flap reconstruction (2,3,4). The free TRAM or DIEP flap also offers the potential advantage of improvement in the contour of the abdominal wall: elimination of soft tissue excess, correction of lower abdominal musculofascial laxity, and repair of epigastric rectus diastasis. Contour abnormalities, however, may result; the rate of donor-site complications such as lower abdominal fascial bulging and even hernia generally speaking increase with the amount of rectus muscle sacrifice and number of previous abdominal surgeries (5,6,7,8,9,10).
Minimization of donor-site morbidity and the gradual evolution of technique to spare muscle sacrifice (from free TRAM to muscle-sparing free TRAM and DIEP flaps) have further improved the balance of risk and benefit in favor of free tissue transfer. However, many factors contribute to implants remaining the preferred reconstructive method across the United States. The need for microsurgical training, the overall complexity of the operation, the mental and physical demands on the operative team, the need for a team approach, and declining reimbursement are all factors that favor the tertiary academic centers or large specialized group private practices, both of which take years to develop. However, identification of the factors contributing to the underutilization of these and other flaps allows for design and implementation of surgical and clinical pathways that increase efficiency and improve clinical outcomes (11).
Indications and Patient Selection
In our practice patients range in age from young to old (28 to 76 years) and in body types ranging from thin to obese (body mass index 18 to greater than 40). Approximately 66% of our patients are obese or morbidly obese. We have performed breast reconstruction in primary, secondary (after previous failed reconstruction), and tertiary (after multiple failed reconstructions) settings; in smokers; in patients with significant past medical histories (typically diabetes and/or hypertension) and significant surgical histories (multiple previous abdominal procedures); following breast and/or chest wall radiation (Figs. 55.2 and 55.3); and even following kidney and heart transplant surgery. While this may read like a laundry list of “who not to operate on,” we feel that microsurgical breast reconstruction offers the best option for these patients as it typically allows for transfer of a larger volume of well-vascularized tissue and is associated with fewer complications than other reconstructive options. Perforator flap reconstruction is now the preferred method of breast reconstruction in our practice due to the lower donor-site morbidity compared to the free TRAM, although tissue expander/implant reconstructions are also occasionally performed in patients who are not candidates for a flap reconstruction, who do not wish to risk donor-site morbidity, or who choose this option over the various flap options.
Contraindications
Absolute contraindications to free tissue transfer include a known history of clotting abnormalities or bleeding diathesis that cannot be corrected medically and prior abdominoplasty with prior elevation of the abdominal apron. Medical comorbidities such as uncontrolled hypertension, poorly controlled diabetes, and a refusal to begin an immediate smoking cessation program are also strong relative contraindications. We have performed abdominal flap procedures on patients who have had multiple abdominal surgeries with resultant suprapubic and subcostal incisions; however, one must realize that measures should be taken to minimize risk, such as limitation of undermining and consideration of the use of preoperative
duplex or computed tomography (CT)–guided imaging of perforators.
duplex or computed tomography (CT)–guided imaging of perforators.
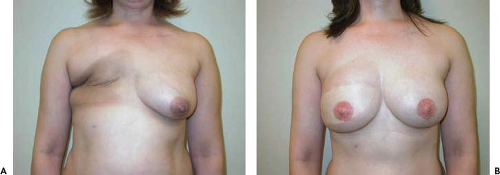
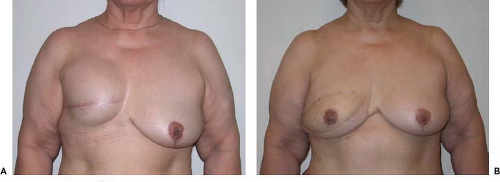 Figure 55.1. Failed right breast implant reconstruction with significant breast asymmetry and attenuation of right breast skin envelope. A: Preoperative. B: Postoperative. |
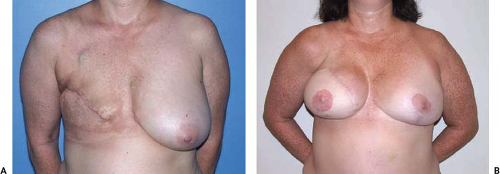 Figure 55.2. Delayed right breast reconstruction following modified radical mastectomy and chest wall radiation therapy. A: Preoperative. B: Postoperative. |
Preoperative Planning and Operative Technique
Successful breast reconstruction depends heavily on an operating room with well-trained, motivated personnel and well-maintained, high-quality equipment. A high priority is placed on assembling surgical technicians and circulating nurses who can perform all methods of breast reconstruction and, especially in our practice, microsurgical flap procedures. Ideally, as the same relatively few personnel participate in flap reconstructions, skill and efficiency improve. Similarly, assembling a small core group of anesthesiologists who are willing and able to perform special tasks during the procedure is helpful. Key functions include establishing and maintaining dense muscle paralysis to permit rapid, safe flap harvest, maintaining adequate hydration to avoid vasospasm, controlling blood pressure, keeping the patient warm, extubating the patient smoothly and painlessly, and preventing postoperative nausea and vomiting.
The precise boundaries of the breast are marked preoperatively with the patient in a sitting position, highlighting the inframammary fold and medial, lateral, and superior extents of the breast parenchyma. Typically, a skin-sparing mastectomy pattern is marked except in cases in which the tumor is located close to the skin, resulting in a larger native skin and soft tissue defect. For patients with grade I to grade II ptosis a vertical reduction pattern is often used. For grade III ptosis a Wise pattern is considered; however, if this pattern is used, care must be taken to keep the native breast skin flaps thick or tissue necrosis will occur in the triangular areas of the skin flap.
One of the members of our group usually assists the general surgeon performing the mastectomy. The general surgeon is encouraged to respect the boundaries of the breast, especially the inframammary fold, and to keep the skin flaps thick enough to preserve perfusion. Following the mastectomy, in cases of free TRAM or DIEP flap reconstruction, flap harvest and exploration and dissection of the recipient vessels proceed simultaneously.
Chest wall preparation begins with assessment of possible bleeding sites from the mastectomy site, as small perforating vessels from the serratus musculature and upper rectus musculature at the inframammary fold are routinely the source of unwanted postoperative bleeding when it occurs. Re-establishing the inframammary fold landmarks and lateral boundary of the breast are frequently necessary following mastectomy in order to achieve the best possible cosmetic result. A drain placed along the fold and in the axilla if nodal dissection was performed minimizes the possibility of postoperative seroma.
We prefer to use the internal mammary vessels to provide inflow to the flap. Once the exposure technique is mastered, these vessels are readily accessible, provide strong arterial inflow, and permit rapid positioning of the flap and a comfortable working position under the microscope. A potential disadvantage is that the left internal mammary vein, or veins, can be thin walled and of small caliber (1 to 1.5 mm), making the venous anastomosis slightly more difficult. Despite this caveat, in our experience the left internal mammary vein can routinely and safely be used for venous outflow. The right internal mammary vein is routinely of adequate caliber (2 to 3 mm). We have also used the second intercostal perforator vessels if they have been left intact during the mastectomy and if they are of good caliber. Use of this recipient pedicle obviates the need for excision of a segment of costochondral cartilage, which saves time and reduces parasternal soreness postoperatively. Other recipient vessels with which we have experience include the thoracodorsal, circumflex scapular, serratus anterior, and descending (pectoral) branch of the thoracoacromial trunk.
The flap is marked prior to prepping and draping of the patient so that the entire trunk is in full view. Previous abdominal surgery is common in our patient population; right subcostal, Pfannenstiel, and lower abdominal midline scars are frequently encountered, singly or in combination. These incisions do not preclude the surgery. Careful preoperative assessment with the patient in the standing and sitting positions, appropriate placement of the flap, and intraoperative decision making regarding flap viability are required. Lower abdominal perforators are also easily evaluated preoperatively in the office with a pencil Doppler in these situations. CT angiography is another modality than can be very helpful preoperatively in patients with complex abdominal surgical histories, although we do not feel that routine use is necessary.
The free TRAM flap involves transection of the entire width of rectus abdominis muscle encompassing multiple perforating vessels supplying the overlying soft tissue ellipse. This may be associated with permanent loss of abdominal muscle function, which is particularly noticeable to active patients and those requiring bilateral procedures. To minimize abdominal wall morbidity, we harvest a muscle-sparing free TRAM or DIEP flap. For the muscle-sparing TRAM flap, inflow to the subcutaneous tissues is based on two to four perforators, taken from the medial and lateral row. The DIEP flap is typically based on fewer perforators (one or two) and contains no rectus muscle; in our experience the midlevel or upper medial perforator frequently includes a large draining vein, ranging from 2.0 to 2.5 mm in diameter. We will not harvest the flap as a perforator flap unless a large draining vein is present.
As the TRAM flap is raised from lateral to medial, perforating branches of the deep inferior epigastric artery are encountered in the dissection. The perforators are marked (Fig. 55.4A) and circumscribed with the tenotomy scissors under loupe magnification or using low-power magnification with the microscope. Occasionally, a single large perforator cannot be identified, and the decision may be made to include several perforators in the flap dissection. If small perforators straddle the rectus muscle and the decision is made to include rectus muscle with the dissection, the muscle must be divided between the two fascicles through which the perforators originate. Hemostats point to two small perforators in Figure 55.4B. The fascia is then opened and the rectus muscle is encountered. Dissection of the rectus muscle proceeds as the muscle is carefully split in the direction of its fibers, taking care to preserve any crossing branches of motor fibers to the medial aspect of the muscle (Fig. 55.4C). The underlying pedicle of the deep inferior epigastric artery and accompanying veins (hemostat in Fig. 55.4D) is visualized and often encircled with a vascular loop. If the decision is made to perform a muscle-sparing TRAM, then muscle must be divided transversely, perpendicular to the direction of its fibers. (Figure 55.4E shows a clamp placed under the rectus muscle placed from lateral to medial awaiting division of muscle fibers.) The overlying rectus muscle will be divided with the bipolar electrocautery to minimize muscle fasciculations. Figure 55.4F
illustrates the segment of rectus muscle included with the flap tissue. To the left on the figure, a perforator is visualized and the cuff of muscle is seen just medial to the additional perforator noted on the right. The size of the segment of muscle included with the TRAM dissection may vary quite widely. As in this instance (Fig. 55.4G) a larger portion of muscle is included with the flap. Two larger branches of the pedicle are arising in a low-lying position and diverge as they enter the subscarpal fat. In this case a larger width of muscle was included with flap dissection; however, both medial and lateral segments of rectus muscle were left in situ. In this way muscle may be sacrificed and fascia may be preserved, allowing primary closure of the fascial defect. Figure 55.4H demonstrates the area of muscle division, and the perforating branches are visualized at the top of the figure. The apparent muscle defect appears much larger than it actually is in the figure and may often be closed primarily under no tension if the fascia is spared.
illustrates the segment of rectus muscle included with the flap tissue. To the left on the figure, a perforator is visualized and the cuff of muscle is seen just medial to the additional perforator noted on the right. The size of the segment of muscle included with the TRAM dissection may vary quite widely. As in this instance (Fig. 55.4G) a larger portion of muscle is included with the flap. Two larger branches of the pedicle are arising in a low-lying position and diverge as they enter the subscarpal fat. In this case a larger width of muscle was included with flap dissection; however, both medial and lateral segments of rectus muscle were left in situ. In this way muscle may be sacrificed and fascia may be preserved, allowing primary closure of the fascial defect. Figure 55.4H demonstrates the area of muscle division, and the perforating branches are visualized at the top of the figure. The apparent muscle defect appears much larger than it actually is in the figure and may often be closed primarily under no tension if the fascia is spared.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree