35 Free-functioning muscle transfer in the upper extremity
Synopsis
 Free-functioning muscle transfer involves the transfer of a muscle from a distant location to replace a lost function.
Free-functioning muscle transfer involves the transfer of a muscle from a distant location to replace a lost function.
 It is a complex procedure that requires a highly motivated patient.
It is a complex procedure that requires a highly motivated patient.
 The procedure involves microvascular anastomoses and neural coaptation.
The procedure involves microvascular anastomoses and neural coaptation.
 Meticulous attention must be paid to proper positioning and tensioning of the transferred muscle.
Meticulous attention must be paid to proper positioning and tensioning of the transferred muscle.
 Postoperatively, a highly structured rehabilitation program is required for a prolonged period, often up to 2 years.
Postoperatively, a highly structured rehabilitation program is required for a prolonged period, often up to 2 years.
 In the appropriate patient, reliably good results can be achieved for patients with otherwise devastating injuries.
In the appropriate patient, reliably good results can be achieved for patients with otherwise devastating injuries.
Introduction
Key points
• This is a complex procedure for use where no simpler alternative is available.
• Meticulous attention to microsurgical technique is required; thrombosis leads to muscle loss.
• Attention to muscle placement and tensioning is also critical.
• This technique is applicable to restoration of finger, thumb, and wrist flexion and extension, elbow flexion and extension, and shoulder flexion.
Historical perspective
After multiple attempts by numerous authors, Thompson1 was able to demonstrate that nonvascularized muscular transfer was possible. The first functioning muscle transfer was reported in 1970 by Tamai et al.,2 who transplanted the rectus femoris muscle in dogs. He was able to provide electophysiological and biomechanical evidence of muscular contraction of the transferred muscle. Since this time, multiple authors have pursued functional muscle transfers throughout the body to replace lost function. Functional muscle transfers have been reported in both the upper and lower limb and also for facial reanimation. In 1973 at the Sixth People’s Hospital in Shanghai, microsurgeons successfully transplanted the lateral portion of pectoralis major into the forearm of a patient with Volkmann’s ischemic contracture.3 This was the first clinical application of free functional muscle transplantation in the upper limb. A good range of finger motion and substantial grip force were reported. In 1976 Ikuta et al.4 performed experimental work demonstrating the efficacy of free functional muscle transfer. They were also able to demonstrate almost normal histological condition of the transplanted muscle. In the same year Harii et al.5 showed that it was possible to transfer muscle to the face for treatment of facial paralysis.
Basic science/disease process
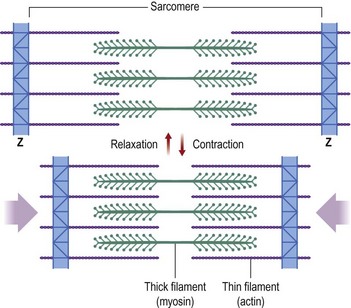
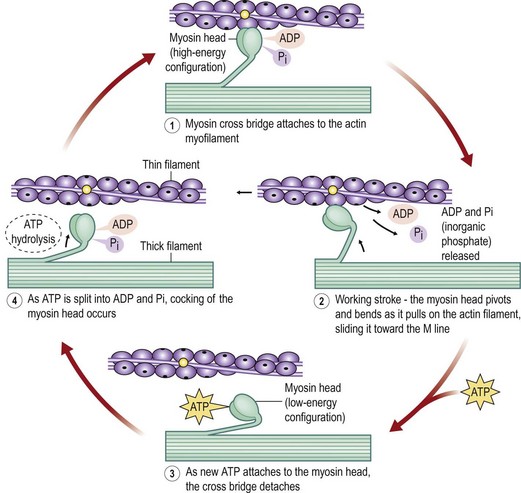
The basic functional purpose of a muscle is to provide both static tension and functional shortening. To understand its functioning, one must understand the structure of a muscle (Fig. 35.1). A skeletal muscle is composed of parallel muscle cells called myofibers. Each muscle cell is composed of smaller parallel myofibrils. These myofibrils are further subdivided into thin and thick filaments containing actin and myosin.6 Within each muscle cell the myofibrils are arranged within functional compartments known as sarcomeres. These sarcomeres are made up of interdigitating fibers of actin and myosin that interact at a subcellular level (Fig. 35.2). When a muscle is stimulated to contract, the actin and myosin fibers move over one another by the flexing action of myosin cross-bridges.7
The amount of overlap of the actin and myosin fibers determines the number of side chains that are in contact and therefore able to contribute to force generation. When a muscle is overstretched there are fewer side chains in contact. When a muscle is overcontracted the myosin chains crumple and cannot contract further.8
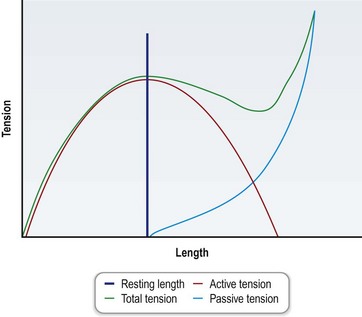
This relationship leads to a length–tension relationship that provides for maximal force generation when the muscle is at its native physiological length (Fig. 35.3). The total force exerted by a muscle is the sum of the passive force provided by the elastic properties of the muscle fibers and surrounding tissues and the active force produced by the contraction of actin and myosin filaments.6 The relationship of length to contractile force generation is described by a bell curve with decreasing ability of force generation further from the physiological resting state corresponding with less overlap of the actin and myosin fibers. For this reason, determination of the resting length of the donor muscle is critical prior to transfer, such that this length can be reconstituted after transfer at inset of the muscle and maximal contractile force can be generated.8
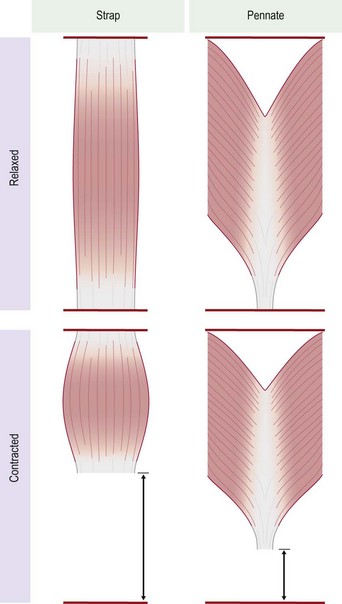
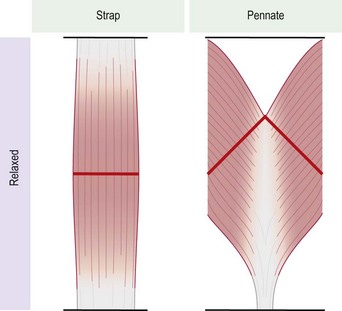
There are many morphological types of muscle in the body. They can be divided into two major types, strap muscles and pennate muscles, or a combination of the two (Fig. 35.4). In a strap muscle the muscle fibers are arranged in parallel to the long axis of the muscle. This is important from a functional standpoint, as the excursion of a strap muscle is directly proportional to its length. It has been demonstrated that at maximal contraction a strap muscle can contract up to 65% of its resting length.6 In a pennate muscle, the muscle fibers are arranged at an angle to a central tendon (Fig. 35.5). As a result, the individual fibers in a pennate muscle usually do not run the entire length of the muscle. The excursion achieved by a pennate muscle is proportional to the length of the muscle fibers, not the length of the entire muscle. As such a pennate muscle will usually yield lesser excursion than a strap muscle.8
The force generated by a muscle is proportional to its total cross-sectional area. Whether this is due to a greater number of muscle fibers or hypertrophy of a smaller number of fibers, the maximal force generation has been calculated in mammals to be approximately 4 kg/cm2.6 The cross-sectional area is calculated perpendicular to the direction of the muscle fibers. In a strap muscle this is perpendicular to the muscle as a whole and perpendicular to the tendon. In a pennate muscle this is perpendicular to the fibers and oblique to the tendon and muscle as a whole, resulting in a greater cross-sectional area and thus a greater potential for force generation.
The contraction of an individual muscle fiber is an all-or-none phenomenon: when a weak contraction is required, only a few fibers within the muscle are stimulated to contract.7 When a forceful contraction is required a greater number of muscle fibers are stimulated to contract. Each muscle is innervated by a motor nerve. The motor nerve joins the muscle via its motor endplates at the neuromuscular junction. A given nerve fiber within the nerve will have a defined number of motor endplates, each of these innervating specific fibers within the muscle. A muscle is thus composed of functional motor units, multiple muscle fibers innervated by the same nerve fiber. Depending on the muscle this may be as few as five muscle fibers such as in the extraocular muscles, or as many as several hundred, as is the case with most major skeletal muscles. This relationship is important in relation to reinnervation and the number of axons provided by a given donor motor nerve. A muscle with smaller muscle units may provide finer control but less power for a given number of axons, and vice versa – a muscle with larger motor units requires fewer axons for greater force generation.
Individual muscles considered for transfer have been assessed with regard to their internal architecture, vascular anatomy, motor innervation, and characteristics relevant to desired function. Ideally the transferred muscle should have a single dominant vascular pedicle (Mathes and Nahai type 1)9 of sufficient length to allow easy anastomosis after transfer without the need for vein grafts. The muscle should possess a single motor nerve of sufficient length and in the correct orientation to allow for primary coaptation of the nerve to the donor motor nerve. The muscle and the limb into which it is to be transferred should possess the appropriate characteristics to allow the muscle to perform its desired function (Table 35.1). These characteristics have been well described for tendon transfers in the upper limb, but apply equally to free functional muscle transfer.
Table 35.1 Principles of tendon transfer applicable to free-functioning muscle transfer
(Adapted from Anastakis D, Manktelow R. Free functioning muscle transfers. In: Green’s operative hand surgery, 5th edn. Philadelphia: Elsevier, 2005.)
Diagnosis/patient presentation
Free functional muscle transfer is applicable to patients who have sustained a major loss of skeletal muscle in the upper limb resulting in a significant functional disability. Free functional muscle transfer is indicated to replace lost function where no easier local option is available. This may be seen following direct trauma, in the setting of Volkmann’s ischemic contracture (Fig. 35.6), following tumor ablation such as sarcoma resection, and in long-standing nerve injury such as brachial plexus lesions. Other rarer indications include electrical burns and post gas gangrene and postreplant patients.10 Each of these indications presents its own special set of considerations.
Of particular note, in the setting of brachial plexus reconstruction, local donor nerves to power the muscle transfer may be limited or completely unavailable. In this case, careful consideration must be given to the choice of donor motor nerve. In some cases, it is necessary to perform nerve grafts as a preliminary procedure to bring viable motor axons into the denervated limb.11,12 A similar picture can be found in the setting of Volkmann’s ischemic contracture; however here the anterior interosseous nerve of the forearm is often spared and can be available as a motor donor nerve.13 This will be discussed further later in the chapter in the section on choice of donor motor nerve.
Patient selection
Probably the most important decision to be made is whether to proceed with free muscle transfer at all. This is a highly involved surgical procedure that requires a motivated patient who is aware of the potential of the procedure and who is fully equipped and prepared to undertake the oftentimes intensive pre- and postoperative rehabilitation that is required for a successful outcome. The patient must understand that the maximal outcome of the procedure will not be realized inside 1–2 years.14 The importance of having a cooperative patient cannot be overemphasized. The ideal patient is stable, intelligent, and motivated to regain function and return to work, with no medical comorbidities. Inappropriate candidates are unfortunately common among patients with injuries who might otherwise benefit from this procedure. Head injuries may often accompany brachial plexus injuries in motor vehicle and motorcycle accidents.
Multiple physical factors must be taken into account prior to muscle transfer. The patient must have stable skeletal structure, including functional joints. He or she must have normal or near normal range of motion in the hand, wrist, and elbow. There must be adequate soft-tissue cover over the planned tendon-gliding site. If there is insufficient unscarred soft tissue to cover the planned site, soft-tissue reconstruction in the form of tissue expansion or local flaps may need to be performed prior to muscle transfer. Sensation in the hand should be normal to achieve optimal results, though this is not an absolute requirement.10 There must be sufficient evidence that there are suitable recipient artery and veins in the arm to vascularize the transferred muscle. Preoperative Doppler ultrasound or angiography can be very helpful in this regard.8 Also a suitable donor nerve must be available to power the transferred muscle. There should be adequate antagonist function in the arm to allow the transfer to be of use, for example in performing a transfer to replace long finger flexor function and restore grip, there must be adequate finger extensor function to oppose the new action of the transferred muscle and to release the grip (Table 35.2).
Table 35.2 Requirements for free functional muscle transfer