5 Facial skin resurfacing
Synopsis
 Non-surgical facial rejuvenation relies on matching the presenting skin pathology to an appropriate intervention that reverses that aspect of aging.
Non-surgical facial rejuvenation relies on matching the presenting skin pathology to an appropriate intervention that reverses that aspect of aging.
 Laser-based interventions rely on precise skin injury based on either selective photothermolysis, precise deposition of layered heating, or fractional wounding.
Laser-based interventions rely on precise skin injury based on either selective photothermolysis, precise deposition of layered heating, or fractional wounding.
 Facial rejuvenation procedures that work at the skin surface can be complemented by neurotoxins and fillers.
Facial rejuvenation procedures that work at the skin surface can be complemented by neurotoxins and fillers.
 Fractional lasers achieve their rejuvenative effect with decreased risks of infection, pigment dyschromia, pain, as well as shorter recovery times versus their nonfractional counterparts.
Fractional lasers achieve their rejuvenative effect with decreased risks of infection, pigment dyschromia, pain, as well as shorter recovery times versus their nonfractional counterparts.
 Vascular dyschromias can be reduced by a wide range of visible light and near infrared technologies.
Vascular dyschromias can be reduced by a wide range of visible light and near infrared technologies.
 Hyperpigmented lesions can be treated by selective photothermolysis, repeated fractional procedures, or by precise ablative approaches that confine heating based on short laser tissue interaction times.
Hyperpigmented lesions can be treated by selective photothermolysis, repeated fractional procedures, or by precise ablative approaches that confine heating based on short laser tissue interaction times.
Introduction
Laser, chemical peeling, and other energy-based technologies have increasingly been applied in facial rejuvenation.1 A logical approach to rejuvenation follows an understanding of skin anatomy and physiology, as they relate to skin aging. Any assessment of the face should include the surface, where sun and aging result in pigment inhomogeneities, wrinkles, and telangiectasia. Epidermal changes include basilar hyperpigmentation, hyperkeratosis, and thinning of the “living” portion of the epidermis. Another component of skin aging derives from changes in the dermis, where decreased glycosaminoglycans (GAGs), decreased elastin fibers, and changes in the character of collagen result in fine lines, sallowness and eventually in cobblestoning of the skin. Microscopically, the changes present as solar elastosis. Also, weakened blood vessels dilate and present as telangiectasia. In some patients, hyperpigmentation results from both dermal and epidermal pigment dyschromias. The third component of aging skin results from bone regression, weakening of connections from the hypodermis to the surface, and volume loss.1,2
Most energy-based interventions address one or more of these components of skin aging. Epidermal pigmentation can be addressed by pigment-specific lasers or laser peels. In the first case, visible (VIS) light is used to selectively heat the epidermis. Proper parameter selection allows for preferential targeting of the hyperpigmented lesion, whereas the normal background “innocent” bystander skin is unharmed. Traditional “nonfractional” laser peels, on the other hand, target water and therefore heat a confluent “slab” of the uppermost skin. The depths of ablation of heating are determined by wavelength, power density, fluence, and pulsewidth.1
Blood vessels are heated by three broad categories of devices: (a) visible light technologies (520–600 nm); (b) near infra red (NIR I) technologies (755, 800 nm); and (c) NIR II (940, 980, 1064 nm). The former is associated with very strong HgB and melanin heating, the second by moderate HgB and melanin heating, and the third by moderate HgB heating but relatively weak melanin heating. Deep heating devices have included laser, halogen lamps, xenon flash lamps with long wavelength cut-off filters, RF, US, and combination technologies.3
The use of light as a medical treatment has grown considerably since the advent of the medical laser in the 1960s. The word laser is an acronym for the term: light amplification by stimulated emission of radiation. The device itself consists of an energy source, a laser medium and a resonating tube. The medium can be a gas, liquid, or solid, and this will often be used to name the type of laser. (Examples: ruby laser and CO2 laser). The light emitted is composed of photons that travel in the same direction, making laser light highly directional. Laser light is monochromatic, which means that all photons have the same wavelength. By contrast, intense pulse light (IPL) consists of many different wavelengths. The specific wavelength of each laser will determine how the laser beam interacts with tissue. The light can be reflected by tissue, scattered by the tissue, or it can transmit through tissue. The intention is that the laser light be absorbed by a specific target tissue (the “chromophore”). The mechanism by which lasers are used to target specific tissue is called selective photothermolysis (photo = light, and thermolysis = decomposition by heat).4
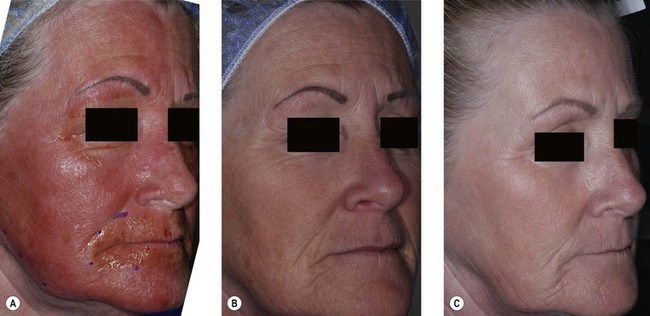
Lasers can be broadly broken into two categories, ablative and nonablative. Until recently, ablative (means “to remove”), lasers have been the “gold standard” of care for wrinkle reduction. The carbon dioxide laser with a wavelength of 10 600 nm and the Er:YAG with a wavelength of 2940 nm are mainstays of ablative laser treatment. A relative newcomer is erbium YSGG at 2790 nm.5 With each of these lasers, an intense burst of energy is delivered onto the skin. The energy heats water in the skin and causes both the water and tissues to vaporize. With each pass of the laser, a controlled depth of skin is vaporized and/or coagulated. In response to the injury and subsequent healing, new layers of collagen are produced. While ablative nonfractional lasers can be very effective and have a firm place in laser skin rejuvenation, each can be associated with risks of infection, scarring, hypopigmentation and unnatural alterations in the texture and sheen of the skin. Moreover, complex aftercare is required until the skin is fully healed. Resolution of erythema may take months. Outside of fractional approaches (vide infra), these lasers are also limited to the thicker skin of the face rather than the thinner skin of the neck and hands.3
Nonablative nonfractional treatments are safer than their ablative counterparts, but require epidermal cooling, which may reduce efficacy of the treatment. Generally, small therapeutic windows are associated with nonablative treatments and outside of dyschromia reduction with visible light approaches, only modest cosmetic enhancement is achieved. The Nd:YAG 1320 nm pulsed laser (e.g., Cooltouch) is an example of a nonablative laser in wide clinical use. IPL (intense pulsed light), monopolar radiofrequency skin tightening, LHE (light heat energy) and LED (light emitting diode) are all examples of nonablative treatments.1–3
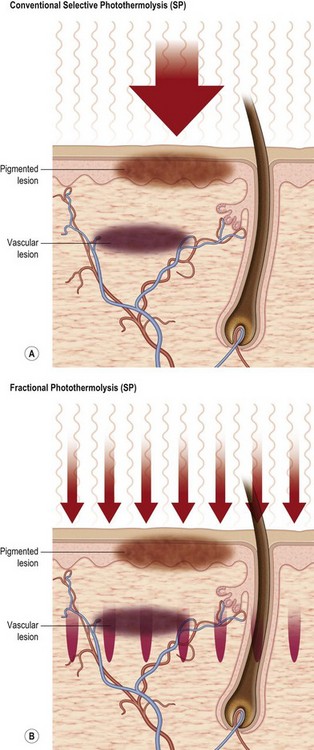
Skin rejuvenation with fractional photothermolysis represents a newer class of therapy (Fig. 5.1). Thousands of microscopic wounds surrounded by viable tissue permit rapid healing and are made with a variety of laser wavelengths and delivery systems. Immediate and delayed therapeutic results are seen through a combination of epidermal coagulation for surface enhancement and dermal heating for deeper remodeling. Unlike selective photothermolysis, in which targets are damaged based on color contrast, fractional photothermolysis only damages specific zones based on the pattern of the micro-beams, leaving other zones completely intact. Fractional laser techniques began with a 1550 nm wavelength. The concept of a fractional laser can be applied, however, to almost any wavelength of light and can be used with both ablative laser resurfacing and nonablative laser rejuvenation. With increasingly aggressive densities and depths of injury, the fractional approach may achieve comparable results to non fractional approaches, without the associated side-effects.4
Fractional photothermolysis was introduced in 2003 as the Fraxel™ SR by Reliant Technologies of Palo Alto, California, now Solta Medical, Hayward, California.4,5 Their fractional laser was cleared by the FDA for periorbital wrinkles (2004), skin resurfacing (2005), melasma (2005), pigmented lesions, freckles, age spots (2004), acne (2006). Since then, a number of other companies have introduced lasers capable of delivering fractionated light. Herein, we review the various laser devices available and discuss our clinical experience with fractional photothermolysis.4,5
Chemical peelings represent accelerated exfoliation or skin damage induced by caustic agents that cause controlled damage, followed by the release of cytokines and inflammatory mediators, resulting in thickening of the epidermis, deposition of collagen, reorganization of structural elements, and increases in dermal volume. This process decreases solar elastosis and replaces and reorients the new dermal connective tissue. The result is an improved clinical appearance of the skin, with fewer rhytides and decreased pigmentary dyschromia.1,3
History of resurfacing procedures
Lasers have been used in medicine for several decades. Alexander et al.1 noted that Albert Einstein was the first to describe the concept behind the laser in the theory of stimulated emission of radiation in 1917.2 Theodore Maimon developed the first laser light with use of a ruby crystal in 1960 and shortly thereafter, lasers were being used in ophthalmology.1 During the next several years, practitioners in many other medical fields, including dermatology, otolaryngology, neurosurgery, and gynecology, incorporated lasers into their practices. Selective photothermolysis, is the theoretical foundation that allowed the development of lasers designed to target specific tissues.4,5 According to Alexander et al.,1 the first of these lasers was the pulsed dye laser, with a wavelength of 577 nm, one of the absorption peaks of hemoglobin. This device was applied primarily for vascular lesions. Alexander1 noted that Leon Goldman, a dermatologist considered by many to be the father of laser medicine, pioneered much of the early work with cutaneous lasers and published his experience in the treatment of vascular birthmarks with the ruby laser.6–8
The CO2 laser, developed in 1964, was initially a continuous wave laser. It was designed with a highly focused handpiece to cut skin.1 Its injury was associated with a bloodless field. It also appeared to generate less edema, possibly because the laser sealed lymphatics.9 Sterling Baker, an ophthalmologist, is credited with having performed the first laser blepharoplasty in 1984.10 The CO2 laser was also used as an ablative tool for cutaneous lesions, but early lasers had limited control of energy parameters, leading to frequent thermal injury and scarring.1
According to Alexander and his co-authors,1 changing the delivery of laser energy from a continuous to a pulsed mode was key in the early improvement of the resurfacing laser. Electronic shutters were developed to interrupt the continuous wave and produce intermittent bursts of laser energy, known as pulses, thereby decreasing the laser exposure time. The original pulses were 0.1–1 s in duration, still too long an exposure time to avoid scar formation unless very low powers were applied.1 Superpulse lasers were then developed, which delivered shorter pulse durations (pulse widths) and higher power, but still led to an unacceptable incidence of complications.1 Ultrapulse technology provided an increase in power seven times that of the superpulse lasers, and the pulse width was decreased to less than 1 ms.1 These early advances helped limit the thermal damage and associated scar formation, allowing the CO2 laser to be used effectively as a resurfacing tool. In 1991, Fitzpatrick et al. used this laser to treat actinic damage of the face and actinic cheilitis and noted improvement in wrinkles.11 These observations led to the use of laser resurfacing with short-pulsed, high-energy CO2 to treat photodamaged skin and acne scars.1
The introduction of short-duration Er:YAG lasers in the mid-1990s offered another option for resurfacing, either alone or in combination with the CO2 laser.1 Most of the initial laser resurfacing research is credited to dermatologists.12 Laser resurfacing grew in popularity and gained widespread acceptance among aesthetic plastic surgeons. As more problems with scarring and hypopigmentation were reported, the early enthusiasm toward ablative CO2 and Er:YAG waned.1
In an effort to expand treatment to all skin types and to improve safety, reducing the frequency of scarring and loss of pigmentation, fractional laser resurfacing was introduced by Reliant technologies in 2003 as a new class of therapy. Fractional photothermolysis has become an important modality in management of a number of skin conditions and photoaging. Unlike full surface flat beam resurfacing, fractional resurfacing damages specific microtreatment zones within the target area. It seems that almost any laser wavelength can be fractionated and the initial nonablative fractional lasers have been safe and effective for treatment of fine wrinkles, pigmentation and acne scars. Another nonablative technique that has gained popularity within the last decade is monopolar radiofrequency skin tightening (Therma-cool radiofrequency, Thermage, Inc.).13 Developed in the mid-1990s and later approved for use by the FDA in 2002, monopolar radiofrequency delivers a controlled radiofrequency pulse to the dermis and sub-dermis to promote collagen remodeling.13 Inadvertent injury to the more superficial dermis is avoided through use of a cryogen spray during application of the energy.13
Deeper rhytids and more extensive solar elastosis have not responded as well to nonablative wavelengths and hence, fractional CO2 and Er:YAG lasers have been developed that retain much of the safety of NFR, but push the efficacy closer to nonfractional laser ablation. Since the introduction of the first fractional laser in 2003, numerous devices delivering fractionated treatment with a variety of different capabilities have appeared on the market. More importantly, in the aesthetic arena, where safety is of paramount importance, fractional laser resurfacing has resonated widely with both patients and physicians alike and has become a leading trend in minimally invasive cosmetic surgery.14–16
Chemical peeling methods have been used to rejuvenate skin since the days of ancient Egypt. Lactic acid is the original peeling agent, which is an active ingredient of sour milk that was used topically by the nobles as part of an ancient skin rejuvenation regimen. In the Middle Ages, old wine with tartaric acid was used for the same purpose.17 Today, these historical chemicals are known to contain alpha hydroxy acids. It is these acids which are the active ingredients responsible for skin exfoliation. Modern-day chemical peeling originally was promoted by dermatologists, such as PG Unna.17,18 He first described the properties of salicylic acid, resorcinol, phenol, and trichloroacetic acid (TCA). Early practitioners of chemical peels began to develop other peeling agents for varying depths of penetration. In the 1960s, Baker and Gordon developed a deep peeling agent, which was able to smooth rhytids and furrows. From the 1980s to the present, scientific investigation was finally undertaken by plastic surgeons and dermatologists, who legitimized their use for cosmetic enhancement of the face.17,18 This included establishing the indications and functional uses of the procedures and improving their safety and efficacy. Currently, there are a number of categories of chemical peeling agents available for rejuvenating the skin. Peeling agents range from superficial formulations available over-the-counter to deep chemical agents are typically only applied by a trained physician in a well-controlled setting. When used with appropriate technique, all of these products have proven successful in improving appearance and quality of facial skin. The goal of chemical peeling is to remove a known, uniform thickness of damaged skin. Complications such as scarring and pigmentary changes are minimized as normal wound healing and re-epithelialization occur.17,18
Basic science of resurfacing procedures
Biology of wound healing following chemical peeling
The aim of most forms of chemical peeling is to provide a controlled injury to the target skin, such that healing potential is maximized while scarring is minimized.18,19 The three main forms of wound healing include re-epithelialization, scar formation, and wound contraction.20
In superficial insults to the skin, the basal layer of epidermis is intact and is capable of proliferating and repopulating the epidermis. This form involves minimal involvement of the underlying dermis and thus no scar formation ensues. Following deeper injuries to skin, wound healing relies on keratinocytes from the wound edge and skin adnexa to migrate and proliferate. Collagen within the dermis is affected and undergoes some change. The migration of keratinocytes from the wound edge begins within hours of injury and involves four phases: (1) Mobilization: epithelial cells immediately adjacent to the wound enlarge, flatten, and detach from neighboring cells and the basement membrane; (2) Migration: as marginal cells migrate, the cells immediately behind them also tend to flatten, break connections, and drift along. Epithelial stream continues until advancing cells contact cells from other side, whereupon motion stops abruptly – a process called contact inhibition; (3) Proliferation/mitosis: fixed basal cells away from the wound edge begin mitosis to replace the migrating cells. The cells that have migrated in turn start to divide and multiply; (4) Differentiation: once the wound gap is bridged by advancing cells from the perimeter, normal differentiation of basal cells occurs. The stimuli for keratinocyte migration and proliferation include loss of cell-to-cell contact, growth factors (EGF, TGF-α, KGF, TGF-β), loss of contact with normal components of a basement membrane (type IV collagen and laminin), and contact with proteins of the provisional matrix (fibrin, fibronectin, type I collagen).19,20
The re-epithelialization is facilitated by a moist environment (the proper dressing), debridement of scabs (fibrin, dead neutrophils and other debris), growth factors, and high concentration of skin adnexa (the face and scalp).19,20
Once contact of keratinocytes occurs and contact inhibition is achieved hemidesmosomes re-form, cells become more basaloid, and cellular proliferation generates a multi-laminated neo-epidermis that is slightly thinner.19,20
Inflammation
Tissue injury leads to parenchymal cell damage and the extravasation of blood constituents. The blood clot that forms re-establishes hemostasis and provides a provisional matrix for cell migration. Vasoactive and inflammatory mediators are then generated by platelets (PDGF), the coagulation cascade, the activated complement pathway (C3a and C5a), and injured or activated parenchymal cells.10 Vasoconstriction begins within seconds of the injury and lasts 10–15 min and occurs as a result of epinephrine being release in to peripheral circulation, stimulation of the sympathetic nervous system and release of norepinephrine. Vasodilatation and capillary leak are mediated by a variety of factors including leukotrienes, prostaglandins, kinins, histamine, and complement factors C3a and C5a. Leukocyte migration is stimulated by components of the extracellular matrix and several inflammatory mediators. Neutrophils cleanse the wound of foreign particles and bacteria and are then extruded with the eschar or phagocytosed by macrophages. In response to specific chemoattractants, monocytes infiltrate the wound and become activated macrophages that contribute to the coordination of wound healing. Specifically, angiogenesis, fibroblast migration and proliferation, collagen production, and wound contraction are all directed by macrophages.19,20
The wound healing process is regulated, in a large part, by the ordered production of cytokines that control gene activation responsible for cellular migration and proliferation and synthetic activities.20
Proliferation
This phase includes epithelialization, angiogenesis, granulation tissue formation, and collagen deposition. Epithelialization was discussed above, in the previous section. Angiogenesis, stimulated by TNF-α, is marked by endothelial cell migration and capillary formation. The migration of capillaries into the wound bed is critical for proper wound healing.19,20 Much of the angiogenesis occurs during the early phase of wound healing and involves the sprouting of endothelial cells from post-capillary venules. The granulation phase and tissue deposition require nutrients supplied by the capillaries, and failure of this to occur results in a chronically unhealed wound. The final part of the proliferative phase is granulation tissue formation. Fibroblasts migrate into the wound site from the surrounding tissue, become activated, and begin synthesizing collagen and proliferate. Platelet-derived growth factor (PDGF) and EGF are the main signals to fibroblasts and are derived from platelets and macrophages. PDGF expression by fibroblasts is amplified by autocrine and paracrine signaling.19,20 Fibroblasts already located in the wound site (termed “wound fibroblasts”) will begin synthesizing collagen and transform into myofibroblasts for wound contraction (induced by macrophage-secreted TGF-β1); they have less proliferation compared with the fibroblasts coming in from the wound periphery. In response to PDGF, fibroblasts begin synthesizing a provisional matrix composed of collagen type III, glycosaminoglycans, and fibronectin.20
Remodeling
This phase is comprised of both wound contraction and collagen remodeling.
Wound contraction is the result of specialized fibroblasts that express α-actin (myofibroblasts) and their interaction with cytokines and the ECM. During the 2nd week of healing, fibroblasts assume a myofibroblast phenotype characterized by large bundles of actin-containing microfilaments along the cytoplasmic surface of the cell membrane. Fibroblasts maintain contact with collagen matrix by integrin receptors.19,20 Contraction requires stimulation by TGF-β, PDGF and myofibroblast-ECM interaction via integrin receptors. As myofibroblasts contract they exert force on the ECM and subsequently the wound margin. Scar remodeling predominates after ~21 days. There is no net increase in collagen content despite an increase in tensile strength. Collagen production continues although at a slower rate, and there is an equal rate of collagen breakdown by collagenases. As the wound matures disorganized fine collagen fibres are replaced with thicker fibres arranged parallel to skin stresses. The percentage of type III collagen gradually decreases, as does the quantity of water and proteoglycans. The duration of the maturation process varies depending on how long the wound remains open.19,20
The edge of migrating epithelium marks transition between inflammation and fibroproliferation. In the center, where the wound is open, chronic bacterial invasion provides persistent stimulus for inflammation. This tissue contains inflammatory cells, a high concentration of immature vessels and the components of a provisional matrix (collagen type I, fibrin and fibronectin).19,20 When the inflammatory response is prolonged, this tissue looks like ‘granulation tissue’. Once epithelium covers the wound, the inflammatory stimulus is eliminated and fibroblasts predominate. Farther behind the migrating epithelium, there are fewer fibroblasts indicating a more mature wound. Epithelial cells appear to be the source of a stimulus for inflammatory cells to undergo apoptosis. However, if the inflammatory phase continues longer than 2–3 weeks, then this stimulus may be lost and hypertrophic scarring may result.
Deeper chemical peels will activate this inflammatory and proliferative reaction, with subsequent collagen remodeling. However, if the peel is performed in a controlled environment under optimal conditions, scarring tends to be minimized as the collagen is re-organized. This reorganization results in skin tightening and can help to diminish the presence of fine wrinkles.17,18
Laser tissue interactions and properties of lasers
An understanding of light-tissue and electrical-tissue interactions allows physicians to expand their laser repertoire and optimize outcomes. Lasers as light sources are useful because they allow for exquisite control of where and how much one heats.21 However, tissue reactions are not intrinsically specific to the heating source. In principle, a large number of nonlaser devices (i.e., intense pulsed light) can be used for heating skin.21 In many cases, laser is simply a way to convert lamplight to a more powerful monochromatic form. With respect to lasing media, there are diode lasers, solid-state lasers, and gas lasers.1 An example of a solid-state laser is the erbium glass laser. These lasers have a solid rod that is pumped by a flash lamp. Miniaturized diode lasers have become popular. Some diode lasers are housed separately from the handpiece and delivered by fiberoptics. Others are configured with the laser diodes in the handpiece. Intense pulsed light devices are increasingly comparable to lasers that emit millisecond (ms) domain pulses. Absorption spectra of skin chromophores are broad, and therefore a broadband light source is a logical approach for certain cosmetic applications.1
Basic parameters for any procedure using light are power, time, and spot size for continuous wave lasers; and for pulsed lasers, the energy per pulse, pulse duration, spot size, fluence, and repetition rate.22 All of these parameters should be considered in characterizing a laser procedure. Energy is measured in joules (J). The amount of energy delivered per unit area is the fluence, sometimes called the dose or radiant exposure, given usually in J/cm2. The rate of energy delivery is called power, measured in watts (W). One watt is one joule per second (W = J/s). The power delivered per unit area is called the irradiance or power density, usually given in W/cm2. Laser exposure duration (called pulse-width for pulsed lasers) is the time over which energy is delivered.1
Fluence is equal to the irradiance times the exposure duration.23 Other important factors are the laser exposure spot size (which for wavelengths from 400 to 1200 nm greatly affects intensity inside the skin), whether the incident light is convergent, divergent, or diffuse, and the uniformity of irradiance over the exposure area (spatial beam profile). The pulse profile, that is, the character of the pulse shape in time (instantaneous power versus time), is another feature that can impact the tissue response.22–24
Molecular basis of light-tissue interaction (LTI)
In any light-tissue interaction, the thermal or photochemical effects depend on the local absorbed energy density at the target. Spatial localization of temperature elevation is possible when: (1) the absorption coefficient of the target exceeds that of surrounding tissue (selective photothermolysis); (2) when the “innocent bystander” tissues are cooled so that their peak temperatures do not exceed some damage threshold;24 or (3) by applying very small (usually <500 µm in diameter) beamlets or microbeams (i.e., fractional methods – vide supra). Localized heating, for example, in telangiectases and lentigines, follows from the relative excess of HgB and melanin, respectively, in the lesions versus surrounding skin. In contrast, “nonfractional” mid-IR lasers spatially confine temperature elevation by using heating and cooling schemes that allow for selective dermal heating.25
Targeting discrete chromophores offers advantages over targeting tissue water, especially where the ratio of light absorption between the chromophore and surrounding tissue is large, (i.e., >10).24–26 For example, at least in lighter-skinned patients, targeting dermal Hgb can be achieved with minimal surface cooling. Whereas cooling is desirable even in these cases, the primary indication is analgesia rather than epidermal protection. Also, the risk of a severe injury to the skin is lessened, as there is no bulk heating. Lastly, because temperature elevations are localized, there is often less pain than with devices targeting ubiquitous tissue water. Thermal injury is determined by time/temperature combinations. Protein denaturation is dependent linearly on exposure time and exponentially on temperature. That is, cell death is more sensitive to temperature than time.24 Most devices for rejuvenation are based on photothermal or “electrothermal” mechanisms, that is, the conversion of light or electrical energy to heat. More recently, ultrasound devices have been applied to tighten the skin.27 Two processes govern all interactions of light with matter: absorption and scattering. The absorption spectra of major skin chromophores dominate laser tissue interactions. If tissues were clear, then only absorption would be required to characterize light propagation in skin. However, the dermis is white because of light scatter (milk is a reasonable model for the dermis with regard to scattering). Scattering is responsible for much of the light’s behavior in the skin (beam dispersion, spot size effects, etc.). The main scattering wavelengths are between 400 and 1200 nm (those where tissue water absorption is poor).1
There are three chromophores of interest in skin (water, blood, and melanin). Water makes up about 65% of the dermis and lower epidermis. There is some water absorption in the UV. Between 400 and 800 nm, water absorption is quite small (which is consistent with our real world experience that visible light propagates quite readily through a glass of water). Beyond 800 nm, there is a small peak at 980 nm, followed by larger peaks at 1480 and 10 600 nm. The water maximum is 2940 nm (erbium YAG).1
Selective photothermolysis (SPT)
With the exception of cases where water is heated, rejuvenation of the skin is based on discrete heating by chromophores of relatively low concentration (i.e., melanin, hemoglobin). Anderson described the concept of selective photothermolysis more than 25 years ago.4 He noted that extreme localized heating achieved with selective photothermolysis relies on: (a) a wavelength that reaches and is preferentially absorbed by the desired target structures; (b) an exposure duration less than or equal to the time necessary for cooling of the target structures; and (c) sufficient energy to damage the target.4 The heterogeneity of the skin with respect to HgB and melanin allows for very selective injury in thousands of microscopic targets.
The thermal relaxation time is the time it takes for a target to cool to a certain percentage of its peak temperature (after laser exposure). Larger targets take longer to cool and therefore spatial selectivity is preserved with a wider range of pulse durations. Even so, as a general rule, assuming adequate fluences are applied, longer pulse durations will result in greater collateral damage. In laser scenarios, we assume instantaneous heating of the target, so that τ is usually thought of as the time for cooling after the pulse.4
Reaction types
Photothermal
Most laser applications rely on heating. Photothermal approaches depend on the type and degree of heating, from coagulation to vaporization. There is a range of effects on tissue based on temperature. Below 43°C, the skin remains unharmed, even for exposures as long as 20 min. Thus, one can exceed body temperature by about 5°C without a measurable change in the skin. The first change is a conformational change in the molecular structure. These typically occur at temperatures from 42–50°C. At higher temperatures, very short times (seconds or in extreme cases (>100°C) less than 1 ms) can induce cell death.1,4
Photochemical
Photochemical reactions are governed by specific reaction pathways, and the most common photosensitizer (PS) in skin is PpIX. This PS is formed by skin cells by the pro-drug, aminolevulinic acid (ALA).1,4
Biostimulation
Biostimulation (aka low level laser therapy) belongs to the group of photochemical interactions. Most biostimulation studies involve low-power lasers and this field continues to be a subject of controversy. Home use devices that use LEDs (light emitting diodes) are now available in a wide range of wavelength ranges. Typical fluences are in the range of 1–10 J/cm2, and normally there is no acute temperature elevation, nor any clinical endpoint.1,4
Cooling
Before the addition of surface cooling, fluence thresholds for efficacy and epidermal damage were often close. Visible light technologies, (especially green–yellow light sources such as IPL, KTP laser, and PDL) are the wavelength ranges where epidermal damage is most likely. The epidermis is an innocent bystander in cutaneous laser applications where the intended targets, such as hair follicles or blood vessels, are located in the dermis.1,4
Beyond visible light (green, yellow, and red) sources, surface cooling also has been employed in NIR and MIR lasers. With NIR lasers, surface cooling is important, but not only because of dermal/epidermal-junction derived epidermal heating. In addition, deep beam penetration may cause catastrophic bulk heating. With MIR lasers (1.32, 1.45, and 1.54 µm), the chromophore is water. It follows that with even very low fluences, surface cooling is imperative. Without cooling or a fractional design, water’s ubiquitous nature in the skin causes a laser-induced top to bottom injury. All techniques are susceptible to operator error and device failure. It follows that as physicians rely more heavily on cooling devices, any lack of their proper deployment unveils the dark side of cooling. Fractured sapphire windows, disabled cryogen spray apparatus, and crimped forced air chiller tubes have all contributed to unintended epidermal injury.1,4
Nonablative facial skin rejuvenation (NSR)
The original concept of NSR was wrinkle reduction by selective dermal heating.28,29 Deeply penetrating mid-IR lasers coupled with surface cooling were designed to “bypass” the epidermis. A slab-like dermal injury was created. Unfortunately, “CO2-like” results were never replicated because dermal heating was either too deep or too mild. Superficial severe dermal heating was almost always associated with epidermal damage. It followed that all of these devices “bypassed” the solar elastosis ultimately responsible for most static wrinkles of the face.1,4
The “nonablative” term has now evolved to include any electrical, light, or ultrasound based intervention with “relative” epidermal preservation.30 In addition to wrinkle reduction, new outcome measures include acne scar improvement, telangiectasia resolution, homogenization of pigment, pore size reduction, skin tightening (jowls, neck, and some extrafacial sites) and “improved” skin tone.28–30
Sadick developed a classification scheme for NSR based on photodamage type. In his paper, type I photodamage consisted of surface irregularities, of which there were three subtypes. Type 1a included rosacea and telangiectases; 1(b) was comprised of pore size and skin roughness; and type 1c included pigmentation changes. Type II rejuvenation included deeper wrinkles and volume loss.30
Fractional resurfacing
Resurfacing with fractional photothermolysis represents a newer class of therapy (Fig. 5.1). When wavelengths of around 1500 nm are used, the stratum corneum is left largely intact as thousands of microscopic wounds surrounded by viable tissue are made with a variety of laser wavelengths and delivery systems. Immediate and delayed results are seen through a combination of epidermal coagulation for resurfacing effect and dermal heating for deeper remodeling.
As mentioned above, fractional laser resurfacing first became commercially available in 2003.31 The initial fractional laser uses an erbium-doped fiber to deliver 3000 infrared (1550 nm) laser pulses per second and targets water as a chromophore. The Fraxel™ SR (Solta Medical, Hayward, CA) creates an array of microscopic treatment zones (MTZs) measuring 50–150 µm in densities ranging from 400 to 6400 MTZ/cm2.31 Each MTZ forms a column of thermally denatured collagen from the epidermis to mid-dermis. In contrast to ablative lasers, fractional lasers coagulate only 20% of the treated skin, sparing islands of viable epidermis and untreated dermis that maintain the skin’s barrier function while speeding re-epithelialization (Fig. 5.2).1,31
Diagnosis and clinical evaluation
Adequate evaluation and photographic documentation of the patient prior to peeling or laser treatment is essential.17,18,31 This assessment includes consideration of the severity of actinic damage, depth and number of rhytides, and need for any additional resurfacing procedures. The patient with deep rhytides and excessive facial skin laxity is likely a better candidate for traditional rhytidectomy. The patient with moderate photodamage and medium rhytides may be a more optimal candidate for one of the many types of resurfacing procedures. Some patients may benefit from both procedures because rhytidectomy typically addresses skin quantity and soft tissue malposition, whereas resurfacing addresses skin quality.17,18 It should be noted, however, that great care should be undertaken when both rhytidectomy and resurfacing are performed concurrently. If rhytidectomy is performed, it is recommended to avoid any resurfacing of the undermined facial skin, as wound healing issues may ensue.17,18
An important tool of the evaluation of the patient for a resurfacing procedure is Fitzpatrick’s scale of sun-reactive skin types. This scale describes patients’ reactions to ultraviolet radiation and existing degree of pigmentation. Type I patients always burn and never tan. Type II patients tan only with difficulty and usually burn. Type III patients tan but sometimes burn. Type IV patients rarely burn and tan with ease. Type V patients tan very easily and very rarely burn. Type VI patients tan very easily and never burn.32 Patients with lighter skin types can expect to undergo peeling or laser treatments with minimal concern for abnormal pigment changes, whereas individuals with darker skin are at higher risk for unwanted hyperpigmentation or hypopigmentation.32 Another helpful classification system is the Glogau33
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree