Facial Paralysis
Julia K. Terzis
Katerina Anesti
INTRODUCTION
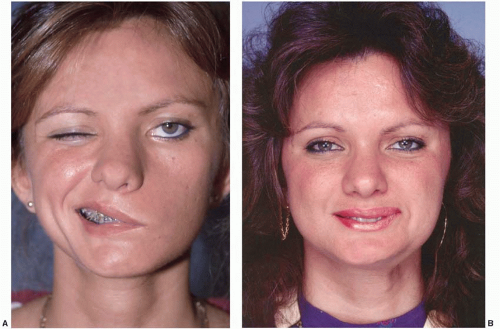
Injury to the facial nerve results in facial paralysis, a devastating condition, as it deprives the afflicted patients of their ability to communicate and express their emotion and negatively affects all aspects of the patient’s life. The subsequent loss of voluntary action of the muscles of facial expression results in facial laxity and a mask-like expression, especially in bilateral cases.
In addition to the aesthetic deformity, there are functional problems related to competence of the eye and oral sphincters causing difficulties with eating, drinking, swallowing, articulation, and speech. Ocular problems include inability to close the eyelids, decreased tear production, loss of blink, and ectropion of the lower eyelid. Speech is variably affected with a degree of dysarthria, as the facial nerve innervates many of the muscles for articulation.
The aim of reconstructive surgery in established facial palsy is to restore symmetry and coordinated animation.
This overview on the most effective reconstructive techniques for reanimation of the unilaterally or bilaterally paralyzed face includes dynamic procedures of neuromuscular rehabilitation, along with supplementary static procedures, which contribute significantly to the overall functional and aesthetic result.
Factors that influence surgical strategies and prognosis in facial reanimation include the patient’s age, denervation time, availability of the proximal facial nerve, whether the paralysis is partial or complete, and availability of motor donors that can be used for reinnervation. Procedures that attempt to restore neural input to a neuromuscular junction give the best results.
The choice of surgical procedures is based on the duration of the facial paralysis, the age of the patient, the cause of the lesion, and the compliance of the patient for a long-lasting and complex rehabilitation program.
Function can be restored by nerve repair or nerve grafting of the facial nerve, or by using the contralateral healthy facial nerve via cross-facial nerve grafts (CFNGs) as long as the time since onset of the palsy is short enough that the paralyzed muscles can still be reinnervated (up to 6 months). Longer denervation times (6 to 24 months) demand the use of a powerful ipsilateral “babysitter” motor donor (for example, partial hypoglossal) that will maintain the musculature, until the CFNGs take over in 9 to 12 months.
For unilateral, irreversible, complete palsy, a three-stage concept is described including CFNGs, free functional muscle transplantation, and several ancillary/revisional procedures.
The role of local muscle transpositions, such as temporalis muscle transfer, and the value of static procedures are also presented.
ANATOMY OF THE FACIAL NERVE
The facial nerve arises from the brain stem nuclei.1 The motor fibers loop dorsally around the abducens nerve nucleus and exit at the cerebellopontine angle (CPA). The parasympathetic and sensory fibers form the nervus intermedius, which join the motor component of the facial nerve as it exits the brain stem.
The course of the facial nerve is divided into six segments: the cisternal segment in the CPA, the intracanalicular segment, the labyrinthine segment, the tympanic segment (separated by the anterior genu where the geniculate ganglion is located), the mastoid segment (separated by the posterior genu), and the extracranial segment. Several branches are given off during the intracranial course.
The geniculate ganglion is the location of the first three branches and mediates parasympathetic functions: the greater petrosal nerve (to the lacrimal gland), the external petrosal nerve, and the lesser petrosal nerve (to the parotid gland).
The next three branches of the facial nerve occur in the mastoid segment: the nerve to the stapedius muscle, a sensory auricular branch, and the chorda tympani, which supplies taste sensation to the anterior two-thirds of the tongue and parasympathetic innervation to the submandibular and sublingual glands. As the facial nerve exits the stylomastoid foramen, it passes anteriorly to the posterior belly of the digastric muscle and lateral to the styloid process of the temporal bone.
The extracranial segment gives off branches to the posterior digastric, stylohyoid, and postauricular muscles, before the nerve splits into the upper (frontozygomatic) and lower (cervicofacial) divisions at the posterior edge of the parotid gland.
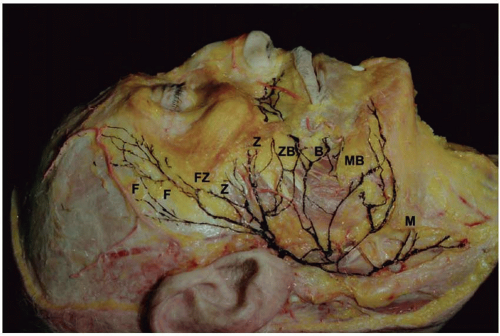
The facial nerve further divides in the parotid gland into several branches. Most commonly, the upper division gives off the frontal, zygomatic, and buccal branches, and the lower division gives off the mandibular and cervical branches. These five terminal branches form a rich plexus that supplies the facial musculature (Figure 36.1).
The facial nerve innervates 23 paired muscles and the orbicularis oris muscle. Seventeen of the paired muscles responsible for facial expression are derived from the mesenchyme of the second branchial arch and are arranged in four layers. The muscles in the three most superficial layers are innervated on their deep surfaces, while the fourth layer muscles (mentalis, levator anguli oris, and buccinator) are innervated through their superficial surface.
ETIOLOGY OF FACIAL PARALYSIS
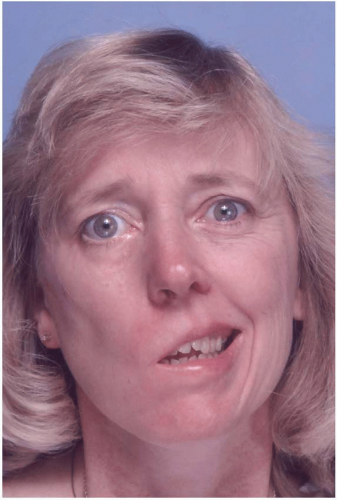
Facial paralysis is a sign or symptom of many disorders, the differential diagnosis of which has been reviewed by May.2 The etiologies can be classified into three major categories: intracranial, intratemporal, and extracranial, depending on the location of the facial nerve lesion. The intracranial causes include vascular abnormalities, brain tumors (CPA tumors being the commonest) (Figure 36.2), developmental abnormalities or agenesis of the facial nerve nuclei, trauma, and/or degenerative disease of the central nervous system. Intratemporally, the causes can be developmental, infectious (bacterial or viral), cholesteatoma, tumors of the middle ear or mastoid area (acoustic neuroma being the commonest), trauma involving fractures of the temporal bone and skull base, or surgery in the region. Extratemporal causes include trauma, malignant tumors of the parotid gland and skin, and iatrogenic.
Idiopathic (Bell’s) palsy is the most common cause of facial palsy, followed by trauma, infections, and tumors. Bell’s palsy resolves in the majority of cases (85%), leaving occasionally some residual weakness in 10% to 15% of patients.
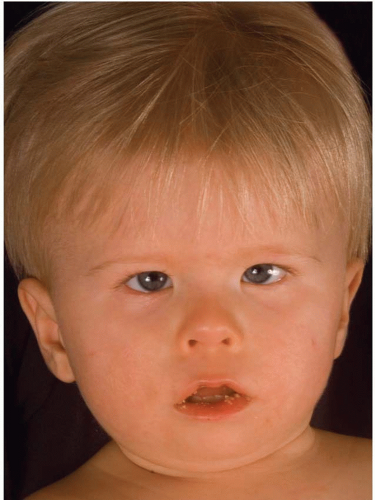
In the pediatric population, facial palsy present at birth should be investigated thoroughly, as the early recognition of developmental facial palsy will lead to appropriate treatment and eliminate long-term sequelae. Congenital facial paralysis refers to conditions that are acquired during or at birth (e.g., from trauma or infection), while developmental facial paralysis (DFP) is the result of anomalies of fetal development. DFP can present in isolation or as part of a recognized syndrome, such as Möbius (Figure 36.3), Goldenhar, and CHARGE.
HISTORICAL REVIEW
Paul of Aegina (626 to 696 AD) was the first to describe repair of divided nerves, while Avicenna introduced epineurial coaptation in the 10th century. Facial nerve surgery developed as a result of research in nerve injuries during the 18th century.
The history of facial nerve surgery can be viewed as five overlapping periods.3 In the first period (1829), Sir Charles Bell demonstrated that the facial nerve innervates the muscles of facial expression and in 1829 described cases of facial paralysis due to trauma. The second period, 1873 to 1960, was the era of facial nerve repair. The focus of facial nerve surgery in the third period, 1908 to 1969, was decompression of the facial nerve.
The fourth period, 1970 to 2000, has been characterized as the “bottleneck” period in honor of the contributions by Ugo Fisch and other surgeons who sought ways to operate on the proximal intraosseous portion of the facial nerve. Scaramella and Smith independently introduced the concept of CFNG, while Anderl popularized its use. The concept brought about new possibilities in restoration of facial expression. In 1976, Harii et al.4 transferred the first gracilis muscle to the face by microneurovascular technique using the deep temporal nerve as the motor donor. O’Brien and Morrison5 recommended the combination of CFNG with microneurovascular muscle transfer, but their use of the extensor digitorum brevis muscle lacked the bulk and power to yield an adequate smile. In 1979, Terzis introduced the pectoralis minor transfer, which subsequently was followed by other authors.6
In the fifth period, 2000 to the present day, further refinements have been made with the introduction of new techniques.
Vascularized nerve grafts are indicated when unfavorable perioperative factors inhibit regeneration (e.g., scarring of recipient bed or radiotherapy). Direct muscle neurotization introduced at the beginning of the 19th century has received recent attention. The use of nerve transfers has also received a following recently (such as the use of the masseteric nerve) for “quick fix” reconstruction. Improvements in surgical outcomes are anticipated especially with microsurgical techniques.
The need for comparison of functional results among different centers in a more standardized fashion is generally expressed.
The need for comparison of functional results among different centers in a more standardized fashion is generally expressed.
AIMS OF RECONSTRUCTION
The aim of reconstructive surgery is to restore symmetry and coordinated dynamic animation with normal appearance at repose, and symmetry during voluntary and involuntary expression, competent ocular and oral sphincters, preservation of existing facial function, and minimal loss of function in other donor motor nerves should be the goal.
PREOPERATIVE EXAMINATION
The history and physical examination are imperative for establishing a management plan. The physical examination includes a complete cranial nerve examination and a careful evaluation of the facial musculature, parotid gland, and neck.
The optimal assessment of the neonate born with unilateral facial paralysis is performed as soon after birth as possible, with the goal to distinguish between a congenital and developmental etiology. It is also important to identify dysmorphic features and multisystem syndromal pathology. May in 19817 provided a list of factors that can aid in differentiating the two forms. The presence of other anomalies and/or bilateral facial paralysis suggests developmental paralysis, while the absence of these signs and the presence of a history of prolonged labor, forceps delivery, hematotympanum, or marks over the ear/mastoid suggest birth trauma.
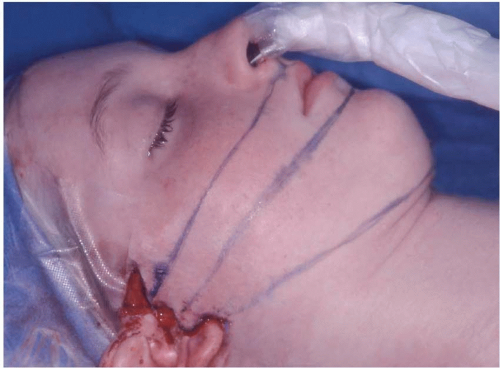
DFP does not improve, whereas traumatic palsy often does. With recovery following trauma, there may be faulty regeneration, yielding synkinesis, spasm, or mass action (Figure 36.4).
A variety of topographic tests exist because of the complex anatomy of the nerve in the CPA and petrosal bone and the fact that the facial nerve is a mixed nerve with motor, sensory, and secretory fibers. To detect the anatomical site of the lesion, tests such as the Schirmer test, stapedius reflex, taste examination, and salivary flow test can be used to assess the severity of nerve degeneration and its evolution over time.7 Electroneurography provides an objective record of evoked compound muscle action potentials and can quantify nerve fiber degeneration. Other studies include needle electromyography (EMG), nerve conduction, blink reflex, and nerve excitability testing. In addition, the high spatial resolution of multisliced spiral computed tomography and magnetic resonance imaging has yielded a more coherent picture of facial nerve disorders. The integrity and dimensions of the osseous facial canal can be delineated along with other central nervous lesions. This is particularly important in the differential diagnosis of neonatal facial palsy.
OPERATIVE TECHNIQUES
Direct Repair
When possible, primary neurorrhaphy is performed. Immediate reconstruction of sharp transections of the facial nerve by primary, direct end-to-end coaptation produces the best results. However, this is only possible in a small percentage of patients. Injuries resulting in long nerve gaps or presence following a significant delay requires alternative techniques, such as nerve grafts, nerve transfers, regional muscle transfers, free tissue transfers, or static procedures.
Epineurial repair is the preferred method for monofascicular nerve stumps, but when dealing with multiple neural segments, perineurial repair is advocated in order to obtain optimal alignment of the severed bundles.
Nerve Grafting
The most common way to overcome a wide neural gap is by the use of autologous nerve grafts (mainly the sural nerve). The average rate of nerve regeneration is 1 to 1.5 mm/d and can be monitored by an advancing Tinel’s sign. Although autologous nerve grafts produce good results, the disadvantages include numbness at the donor site, leg scars, inadequate size match of donor and recipient nerves, and nerve suture sites. In addition, if executed in the intraosseous part of the facial nerve, aberrant motor activities (synkinesis) in selected mimetic muscle groups are frequent occurrences.
Cross-Facial Nerve Graft
Alternative methods of reconstruction of neural defects are required in two occasions: when there is loss of the proximal nerve stump and loss of the distal nerve stump and/or muscles of facial expression.
When the proximal nerve stump is unavailable, the contralateral unaffected facial nerve can be utilized to serve as donor nerve, by borrowing axons from selected branches. These are then connected with nerve grafts that cross the face to reach the affected side, thereby providing motor axons from the normal to the affected side. With this method, coordinated facial motion can be achieved, with the fibers from the intact facial nerve functioning as “pacemakers” for the affected side (Figure 36.5). In a second stage, 9 to 12 months later, the axons in the CFNGs are connected to branches of the affected facial nerve or reach the facial muscle targets directly (direct neurotizations) if the distal nerve stumps were unavailable. The latter requires that the preoperative needle EMGs indicate that some muscle fibers are still present (fibrillations or occasional potentials). If needle EMGs are totally silent, direct neurotization cannot be performed and muscle substitution is recommended, with the CFNG fibers innervating the new muscle unit.
Nerve Transfers
Requirements for nerve transfers8 include a) unavailability of the proximal facial nerve stump, b) intact distal nerve, c) viable facial muscles, and d) inability to use the contralateral facial nerve as a motor donor (e.g., in Möbius syndrome). The ideal time window is determined by the availability of facial musculature. The major disadvantage is loss of function of the donor cranial nerve unless end-to-side coaptation is used. Extensive preoperative electrophysiological testing of all possible motor donors is necessary prior to nerve transfer surgery.
The Principle of “Babysitters”
Although the concept of CFNG is ingenious, it necessitates a prolonged denervation period of the affected facial muscles while regeneration and elongation of the contralateral axons take place. This could lead to irreversible muscle atrophy, unless the CFNG procedure is undertaken soon after the facial nerve injury (within the first 6 months).
For later cases (over 6 months to 2½ years), Terzis in 1984 introduced the “babysitter” procedure.9 This is a twostage procedure (Figure 36.6): the first stage involves the use of 40% of the ipsilateral hypoglossal nerve, which provides powerful motor fibers to the affected facial nerve, reaching target connectivity quickly, and therefore preserving the facial muscle bulk. At the same time, several CFNGs are placed, which are connected to selected branches of the unaffected facial nerve. The second stage, usually 9 to 12 months later, involves secondary microcoaptations between the CFNGs and
selected distal branches of the affected facial nerve. Variations of the “babysitter procedure” have been reported,10 including techniques such as end-to-side grafting11 and concomitant CFNG and hypoglossal facial grafting using a single sural nerve graft.12
selected distal branches of the affected facial nerve. Variations of the “babysitter procedure” have been reported,10 including techniques such as end-to-side grafting11 and concomitant CFNG and hypoglossal facial grafting using a single sural nerve graft.12
Direct Neurotizations
When there is no peripheral stump, direct neurotizations to the muscle target13,14 can take place, provided that the period elapsed is no more than 2 years and preoperative EMG yields fibrillations. In direct muscle neurotization, the contralateral facial nerve or other ipsilateral motor donors (such as part of the hypoglossal or masseteric, or ipsilateral C7 root, or part of the accessory nerve) can be used.15
Muscle Transposition
In long-standing facial paralysis, introduction of a new muscle is required. For older less demanding patients or those who are not candidates for lengthy surgery and prefer a one-stage procedure, a regional muscle such as the temporalis can be used.16
The transferred muscles are innervated by cranial nerves other than the facial nerve. Thus, coordinated movements are not produced and require the patient’s conscious efforts to activate the muscle. Extensive re-training and biofeedback in motivated patients can lead to some degree of coordinated movement.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree