Keywords
Etanercept, Enbrel, TNF-α, OBSERVE-5, Biologic
Key points
- •
Etanercept is a soluble human dimeric fusion protein that competitively binds and inhibits tumor necrosis factor.
- •
Etanercept improved psoriatic skin lesions in phase 2 and phase 3 clinical trials.
- •
Etanercept can be used in pediatric and geriatric populations and is pregnancy category B.
- •
The most common side effects of etanercept are injection-site reactions and infection.
Introduction
Etanercept, a tumor necrosis factor (TNF) inhibitor, was a significant advancement in treatment of moderate-to-severe psoriasis. A quantum leap forward, etanercept provided a combination of efficacy and short- and long-term safety that was unmatched by the other available options at that time, better than systemic therapies, such as methotrexate (MTX), cyclosporine, and psoralen and UV-A radiation (PUVA). Etanercept was the first TNF inhibitor approved by the US Food and Drug Administration (FDA) for plaque psoriasis (May 2004) and is also approved for the treatment of rheumatoid arthritis (RA), polyarticular juvenile idiopathic arthritis, psoriatic arthritis, and ankylosing spondylitis. It is effective for erythrodermic and pustular psoriasis as well.
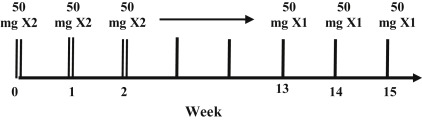
The current recommended dosing of etanercept is 50 mg twice weekly (BIW) for the first 12 weeks, followed by a 50-mg weekly maintenance dose ( Fig. 8.1 ). If the patient prefers, doses of 25 mg weekly or 50 mg weekly are efficacious as well. Etanercept is effective in the pediatric psoriasis population, at a dose of 0.8 mg/kg (up to a maximum of 50 mg) once weekly. Etanercept is available as a single-use prefilled syringe and a single-use prefilled SureClick autoinjector, which patients may subcutaneously self-inject after appropriate training if deemed capable by the dermatologist.
Recommended injection sites for etanercept include the abdomen (excluding a 2-inch circumference surrounding the navel), the front of the middle thighs, and the outer upper arms. Injection sites should be rotated each time, and areas of bruised or tender skin, or psoriatic lesions, should be avoided.
The half-life of etanercept is 4.3 days, the shortest of all the TNF inhibitors approved for psoriasis, which is a potential advantage in the setting of adverse events (AEs). Concentrations peak in the system at 48 to 60 hours with a bioavailability of 58%. Metabolism of etanercept is not decreased in the setting of renal or hepatic impairment, and no dose adjustment is needed when administered alongside MTX, warfarin, or digoxin.
Mechanism of Action
Etanercept, a fully soluble, human dimeric fusion protein, functions as a TNF inhibitor by competitively binding to TNF and preventing its activation of the inflammatory cascade. Etanercept is composed of 2 extracellular ligand-binding domains of the TNF p75 receptor linked to the Fc portion of human immunoglobulin G1 (IgG1) by 3 disulfide bonds. Etanercept is a soluble form of the p75 receptor that inhibits TNF-α, and to some extent TNF-β, by blocking its interaction with cell-surface TNF receptors. Etanercept is different from adalimumab and infliximab, which are monoclonal antibodies to TNF. The dimeric structure of etanercept allows it to bind TNF at an affinity that is 50 to 1000 times greater than naturally occurring TNF receptors ( Fig. 8.2 ).
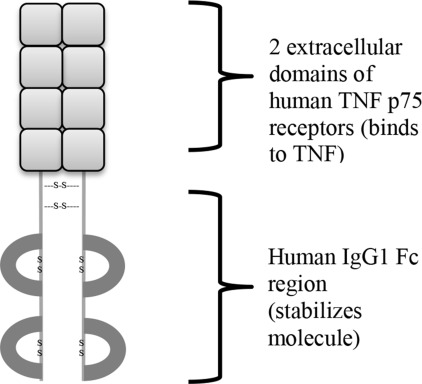
Etanercept is a very large, complex glycoprotein. A batch of etanercept contains multiple variants with subtle differences; those variants can vary from batch to batch. Etanercept is a mixture, and the mixture changes over time. Understanding that there is variation in the brand name etanercept product may be important in considering how etanercept biosimilars should be perceived.
By binding and sequestering TNF, etanercept modulates biologic responses involved in the pathogenesis of psoriasis, such as the expression of adhesion molecules that function in leukocyte migration (E-selectin and intercellular adhesion molecule-1), serum cytokine levels, and serum matrix metalloproteinase-3 levels.
Efficacy
Etanercept therapy improves psoriatic skin lesions considerably. Patients may show some loss of response after 12 weeks of therapy with the recommended step-down from 50 mg BIW to 50 mg weekly dosing. Clearance is better maintained with uninterrupted therapy, although rebound does not typically occur with discontinuation of therapy. There is a potential for loss of efficacy of etanercept therapy over long periods of time, possibly because of antibody development or poor adherence.
Pivotal Trials
US psoriasis pivotal trial
A 24-week, phase 3, double-blinded study across 47 sites in the United States assessed the clinical response of etanercept monotherapy in patients with psoriasis ( Fig. 8.3 ). The primary measure of efficacy was the percentage of patients in each of the treatment groups who achieved a Psoriasis Area Severity Index (PASI) 75 score by week 12. The study population consisted of 652 patients with a mean psoriasis duration of 18.7 years, a mean affected body surface area (BSA) of 28.7%, and a mean baseline PASI of 18.4.
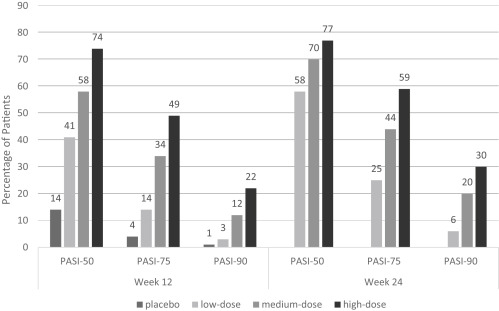
During the first 12 weeks of this study, patients were randomized to receive either placebo, low-dose etanercept (25 mg weekly), medium-dose etanercept (25 mg BIW), or high-dose etanercept (50 mg BIW), in order to assess dose-dependent responses to therapy. At week 12, the placebo group then began to receive medium-dose etanercept as well.
By week 12, PASI-75 was achieved by 4% of patients in the placebo group, 14% of patients in the low-dose group, 34% of patients in the medium-dose group, and 49% of patients in the high-dose group ( P <.001 for all compared with placebo). Statistically significant differences were noted from the placebo group as early as week 4 in the high-dose group, and week 8 in the medium-dose group. Mean levels of improvement as assessed by PASI score in the 4 groups by week 12 were 14.0% in the placebo group, 40.9% in the low-dose group, 52.6% in the medium-dose group, and 64.2% in the high-dose group.
At week 24, PASI-75 was achieved by 25% of the low-dose group, 44% of the medium-dose group, and 59% of the high-dose group (there was no placebo control in the second half of this study). From the original placebo group, 33% of patients had achieved PASI-75 after crossover to etanercept therapy starting week 12, consistent with the medium-dose group at week 12 (34%).
This pivotal trial demonstrated significant dose-dependent increases in etanercept efficacy after 12 weeks of therapy, and continued improvement in psoriatic skin lesions with continued therapy. An extension of this study aimed to determine whether etanercept could be used in an intermittent treatment paradigm by assessing how long psoriasis stayed under control after withdrawal of therapy, and once relapsed, whether control of psoriasis could be re-established with reinitiation of therapy. Patients from the initial study who achieved at least PASI-50 by week 24 discontinued etanercept therapy until they experienced relapse of disease (loss of ≥50% of week 24 PASI improvement). They were then reinitiated on etanercept at their originally randomized dose (25 mg BIW or 50 mg BIW or 25 mg weekly). Patients relapsed on average 3 months after withdrawal from etanercept therapy. After 12 weeks of re-treatment, efficacy data were similar to that of the initial 12 weeks ( Table 8.1 ). Re-treatment of psoriasis with etanercept appeared effective and well-tolerated.
| US Pivotal Trial | Global Pivotal Trial | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Week 12 | Week 24 | Week 12 | Week 24 | ||||||||||
| Placebo | LD | MD | HD | LD | MD | HD | Placebo | 25 mg BIW | 50 mg BIW | Placebo | 25 mg BIW | 50 mg BIW | |
| Injection-site reaction | 7 | 11 | 17 | 13 | 14 | 20 | 16 | 6 | 13 | 18 | 10 | 5 | 4 |
| Headache | 7 | 3 | 12 | 7 | 5 | 12 | 9 | 8 | 12 | 11 | 3 | 5 | 6 |
| Upper respiratory infection (URI) | 11 | 10 | 9 | 5 | 14 | 14 | 12 | 13 | 13 | 13 | 16 | 16 | 13 |
| Accidental injury | 4 | 4 | 3 | 4 | 7 | 7 | 7 | 6 | 4 | 7 | 6 | 4 | 5 |
| Flulike syndrome | — | — | — | — | — | — | — | 2 | 5 | 4 | 2 | 6 | 3 |
| Sinusitis | 1 | 0 | 0 | 0 | 6 | 6 | 5 | — | — | — | — | — | — |
| Myalgia | 2 | 2 | 4 | 2 | 5 | 7 | 4 | — | — | — | — | — | — |
| Nausea | 1 | 3 | 2 | 2 | 5 | 3 | 3 | — | — | — | — | — | — |
| Rash | 2 | 3 | 2 | 3 | 2 | 4 | 6 | — | — | — | — | — | — |
| Asthenia | 3 | 4 | 4 | 2 | 6 | 7 | 3 | — | — | — | — | — | — |
Global psoriasis pivotal trial
Etanercept was tested in a 24-week, global phase 3 randomized controlled trial across 50 sites in the United States, Canada, and Western Europe to further investigate the efficacy after dose reduction ( Fig. 8.4 ). The primary endpoint in this study was achievement of PASI-75 after 12 weeks of study. Secondary efficacy endpoints at week 12 were achievement of PASI-50 and PASI-90, and percentage improvement from baseline PASI. The study population consisted of 583 patients with a median duration of psoriasis of 19 years, median BSA affected of 23%, and median baseline PASI of 16.4. During the first 12 weeks of this study, patients were randomly assigned to receive placebo, etanercept 25 mg BIW, or etanercept 50 mg BIW. During the second 12 weeks, all study patients received etanercept 25 mg BIW.
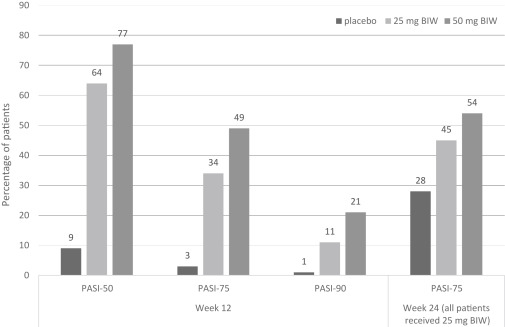
By week 12, 49% of patients in the etanercept 50-mg BIW group and 34% of patients in the 25-mg BIW group achieved PASI-75, in comparison to 3% of patients in the placebo group ( P <.0001). Subgroup analyses based on baseline covariates, such as PASI, age, sex, race, prior systemic or phototherapy, and BSA, revealed no significant impact of these factors on treatment efficacy. Statistically significant differences in PASI-75 responses were seen as soon as week 4 between the etanercept 50-mg BIW group (10%) and placebo group (2%), and as soon as week 8 between the etanercept 25-mg BIW group (20%) and placebo group (3%). By week 12, the mean percentage improvement from baseline PASI was 68% in the etanercept 50-mg BIW group, 57% in the 25-mg BIW group, and 0.2% in the placebo group.
By week 24, PASI-75 was achieved by 54% of patients in the group following a dose reduction from 50 mg BIW to 25 mg BIW, 45% of patients continuing on 25 mg BIW, and 38% of patients that received 25 mg BIW after the initial 12 weeks of placebo treatment. Remarkably, of the 88 patients that had not achieved PASI-75 by week 12, 28 (32%) of them did achieve this by week 24.
This pivotal trial demonstrated statistically and clinically significant dose-dependent improvements in psoriasis with etanercept therapy. This study was also the first to systematically examine the maintenance of etanercept efficacy following a dose reduction and to have found that most patients were able to maintain PASI-75. The results of this study are similar to the findings of Leonardi and colleagues, whose study was conducted with the same doses.
Efficacy in Pediatric and Geriatric Populations
Etanercept is effective in children with moderate-to-severe psoriasis. During a 48-week study in which pediatric patients were given 0.8 mg/kg weekly of etanercept, 57% of patients achieved PASI-75 by week 12 as opposed to 11% of patients receiving placebo. From weeks 12 to 36, all patients received open-label etanercept, and PASI-75 response rates after 36 weeks were 68% and 65% for patients initially randomized to etanercept and placebo, respectively. During weeks 36 to 48, withdrawal effects were studied when patients were randomly reassigned to receive etanercept or placebo. Forty-two percent of patients in the placebo group lost adequate response after withdrawing etanercept therapy, but reached similar response rates as the initial etanercept group with 4 to 8 weeks of re-treatment. A continuation of this study as a 5-year open-label extension study demonstrated that PASI-75 and PASI-90 scores were achieved and maintained in approximately 60% to 70%, and 30% to 40% of the pediatric patients through the 5 years, respectively.
Etanercept is also effective for psoriasis in patients aged 65 years or older, with PASI-75 achieved by 54.1% of patients at 12 weeks, 78.7% of patients at 24 weeks, and 83.6% of patients after 156 weeks of treatment.
Efficacy with Combination Therapy
Combination therapy of systemic and/or topical agents with etanercept has the potential to maximize efficacy, particularly in patients who have failed monotherapy etanercept or who lost adequate response after dose reduction.
Corticosteroids can increase the speed of therapeutic response in patients using etanercept, and the European Dermatology Expert Group recommends concomitant use, especially at the start of therapy.
The UNITE (Utilization of narrowband ultraviolet light B therapy and etanercept for the treatment of psoriasis) study showed that narrowband-UV-B light therapy 3 times weekly combined with etanercept 50 mg BIW in moderate-to-severe patients with psoriasis yielded a PASI-75 response in 84.4% of patients after 12 weeks. The combination therapy in this study was well tolerated, without an increase in photosensitivity, although caution should still be taken in patients with a known or suspected history of skin cancer.
Studies focusing on the combined use of etanercept and MTX have mostly been conducted in RA and psoriatic arthritis patients. The TEMPO (Trial of Etanercept and Methotrexate with Radiographic Patient Outcomes) and COMET (Combination of methotrexate and etanercept in active, early, moderate to severe rheumatoid arthritis) trials both found combination therapy to be generally safe and well tolerated. Zachariae and colleagues studied the combined use of these 2 agents in plaque patients with psoriasis who previously failed MTX monotherapy. They found higher proportions of patients to achieve PASI-50/75/90 and Physician Global Assessment scores of clear or almost clear in the combination therapy group as opposed to the etanercept/MTX taper groups, with little differences in AEs.
Data on the combined use of acitretin and etanercept are limited to case series showing no adverse effects ; thus, further long-term studies are needed to gain conclusive evidence. A pilot trial showed that PASI-75 responses after 24 weeks was achieved in 45% of patients treated with etanercept 25 mg BIW, 30% of patients treated with 0.4 mg/kg acitretin daily, and 44% of patients treated with etanercept 25 mg once weekly plus acitretin 0.4 mg/kg daily. The combined use of acitretin and etanercept may be useful in patients looking to take decreased doses of etanercept.
Safety
General safety concerns with the use of etanercept therapy include infection (bacterial, viral, and fungal), neurologic disease (multiple sclerosis, MS), heart disease, drug-induced lupuslike syndromes, lymphoma (although the risk of lymphoma may be more closely related to having an immune disease and not a direct effect of the drug), melanoma and nonmelanoma skin cancer (NMSC), hematologic disease, and more. Etanercept is pregnancy category B and is considered safe in pregnant women if used with caution. The most common side effect of etanercept is injection site reaction, occurring in up to 37% of patients. These reactions include erythema, itching, hemorrhage, pain, and/or swelling, which are generally mild to moderate and do not require discontinuation of the drug. Precaution should be taken in latex-sensitive patients, because the needle cover of the prefilled etanercept syringe contains latex.
Clinical Trial Safety Data
In the US pivotal trial safety analysis, etanercept was generally well tolerated, with similar AE and infection rates across all treatment dose groups and no reported cases of tuberculosis (TB) or opportunistic infection (see Table 8.1 ). Twenty-seven patients withdrew because of AEs, and 16 patients withdrew because of inefficacy. The global pivotal trial safety analysis also found similar rates of AEs and infections in the treatment groups over 24 weeks. Two patients withdrew from the study because of infection and 9 patients discontinued because of AEs, 5 of which were thought to be drug related.
In the pediatric population, Paller and colleagues reported 3 serious AEs, including an ovarian cyst that required surgical removal, gastroenteritis complicated by dehydration, and serious pneumonia in an asthmatic patient that required discontinuation of etanercept therapy. In the 5-year open-label extension study, no new safety concerns were reported in 181 patients that participated. Through 264 weeks, 89% of patients experienced an AE, most commonly upper respiratory infections (37.6%), nasopharyngitis (26%), and headache (21.5%). There were 8 serious adverse events (SAEs) in 7 patients, only 1 of which (cellulitis) was considered treatment related.
Esposito and colleagues reported a favorable risk:benefit profile of etanercept therapy in the geriatric population. Of the 15 out of 61 patients who withdrew from the study, 2 cases were due to AEs: 1 case was due to repeated episodes of tachycardia after etanercept administration, and 1 case was due to gastric cancer.
OBSERVE-5 Observational Registry Data
The OBSERVE-5 (Observational postmarketing safety surveillance registry of etanercept for the treatment of psoriasis) FDA-mandated phase 4 surveillance registry assessed long-term safety and effectiveness of etanercept therapy in 2510 patients across 375 sites for 5 years. Patients with moderate-to-severe psoriasis were assessed at 6-month intervals for up to 5 years for SAEs, serious infectious events (SIEs) including any infection requiring hospitalization, and events of medical interest (EMIs).
SAEs were those that were fatal, life-threatening, required hospitalization, resulted in disability/incapacity, or were a congenital anomaly/birth defect or a significant medical hazard. EMIs included malignancies (including basal cell and squamous cell carcinomas), TB, opportunistic infections, central nervous system (CNS) demyelinating disorders, lupus disease, coronary artery disease, and worsening psoriasis.
Of the 2510 patients, 418 reported an SAE during the study ( Table 8.2 ). The most common noninfectious SAEs were myocardial infarction (MI; 0.7%), coronary artery disease (0.6%), and osteoarthritis (0.6%). There were 120 patients that reported an SIE, the most being pneumonia (1.2%) and cellulitis (0.9%). There were 604 patients that had 1 or more EMI, 159 of which were considered to be etanercept related.